Testing and Surveillance
One of the most critical aspects of an effective COVID-19 response is public health disease surveillance—the systematic collection, analysis, and interpretation of data to identify cases, map and explain disease spread, and inform the development and implementation of interventions to contain outbreaks. Testing capacity is a key component of effective surveillance systems.

CONTENTS
QUICK DOWNLOADS
In 2020, Exemplars in Global Health launched a series of short- and long-term research projects to help understand the impact of the COVID-19 pandemic in countries and communities around the world, inform COVID-19 response strategies going forward, and apply these lessons to future pandemics.
This short-term research project aimed to identify, document, and explain the testing and surveillance strategies adopted in four low- and lower-middle-income countries in sub-Saharan Africa: the Democratic Republic of the Congo, Nigeria, Senegal, and Uganda. The research has been led by partners at the Makerere University School of Public Health in collaboration with the University of Kinshasa, Université Cheikh Anta Diop, and the University of Ibadan and was supported and funded by the Bill & Melinda Gates Foundation Africa Team and by Gates Ventures.
We aimed to answer the following key research questions for each component of the five-part framework below.
- To what extent did testing and surveillance fit into the country’s overall COVID-19 response strategy, and what were the goals of its testing and surveillance programs?
- How did the country use diagnostic tests to identify COVID-19 cases across six elements of the value chain?
- How did the country collect, analyze, and use data to identify and understand trends in its COVID-19 outbreak or outbreaks? What surveillance modalities did it use?
- What public health actions did the country take because of these data?
- How did the country coordinate its pandemic response from strategy to action?
Methodology
This was a mixed-methods observational study. After researchers established a framework to lay out the testing value chain and role of surveillance in determining public health actions, the first phase of the study involved review of country guidelines and response plans, response reports, websites, meeting minutes, presentations, and analysis of the COVID-19 epidemic curve. Next, researchers conducted key informant interviews with policy makers, program managers, and implementers to further explore the issues identified in the desk research. Finally, the Makerere University School of Public Health synthesized the findings and emerging thematic areas and compiled promising practices, innovations, and challenges into a report with key recommendations for moving forward.
What Do We Know about Testing for COVID-19?
No country, high- or low-income, knows how many of its people have been infected with SARS-Cov-2 (the virus that causes COVID-19) ; only the infection status of those who have been tested is known. Confirmed cases reported by most countries represent lab-confirmed infections. Consequently, testing is essential for all countries to count confirmed cases and understand how the virus that causes COVID-19 is spreading. Without the data testing provides, countries and health systems have no way of understanding how to control the pandemic. Testing data also include information about how much testing a country is actually doing, which allows researchers, policy makers, and others to understand which countries are performing well and which countries may be underreporting or not capturing cases and deaths.
There are three types of tests for COVID-19: molecular, antigen, and antibody. Around the world, but especially in lower-resource countries, molecular reverse transcription polymerase chain reaction (RT-PCR) tests tend to be the most commonly used diagnostic tool. The RT-PCR test is considered the gold standard for confirming a COVID-19 case. The countries we studied did not frequently use antigen and antibody tests during the period of study (November 2020 to March 2021); RT-PCR tests are therefore the focus of this research.
Types of COVID-19 Tests
| Molecular Test | Antigen Test | Antibody Test | |
|---|---|---|---|
Diagnostic test, viral test, nucleic acid amplification test (NAAT), reverse transcription polymerase chain reaction (RT-PCR), loop-mediated isothermal amplification (LAMP) test | Diagnostic test, viral test, rapid test | Serological test, serology blood test, serology test | |
How is the sample collected? | Swab samples from the nose or throat (nasal, nasopharyngeal, oropharyngeal, sputum) or saliva samples | Swab samples from the nose or throat (nasal, nasopharyngeal) | Blood or saliva samples |
How is it used? | Diagnoses active COVID-19 infection | Diagnoses active COVID-19 infection | Shows previous infection with COVID-19; more commonly used for surveillance and research than diagnosis |
How long until results are available? | Less than an hour, same day, or 1–3 days. Some tests may take longer depending on testing capacity and transportation needs | As fast as 15–30 minutes depending on the test | Same day or 1–3 days for tests sent to a lab for processing |
US Food and Drug Administration (FDA). Coronavirus Disease 2019 Testing Basics. Silver Spring, MD: FDA; 2021. Accessed August 12, 2021. https://www.fda.gov/media/140161/download
Antibody (serology) testing for COVID-19: information for patients and consumers. US Food and Drug Administration website. Updated May 19, 2021. Accessed August 12, 2021. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/antibody-serology-testing-covid-19-information-patients-and-consumers
FDA issues Emergency Use Authorization for CovAb™ SARS-CoV-2 Ab Test, the oral fluid rapid test for SARS-CoV-2 antibodies. PR Newswire. June 24, 2021. Accessed August 12, 2021. https://www.prnewswire.com/news-releases/fda-issues-emergency-use-authorization-for-covab-sars-cov-2-ab-test-the-oral-fluid-rapid-test-for-sars-cov-2-antibodies-301318887.html
Coronavirus disease 2019 testing basics. US Food and Drug Administration website. Updated April 7, 2021. Accessed August 12, 2021. https://www.fda.gov/consumers/consumer-updates/coronavirus-disease-2019-testing-basics
Accurate tests for COVID-19 have been available since January 2020, when the first RT-PCR tests were developed. However, challenges can occur at each step of the testing value chain. Over the course of the pandemic, the acuity of RT-PCR tests has varied at different times and in different places. Additionally, many countries have struggled to acquire enough of these tests and long turnaround times for results (often exceeding the length of active infection) continue to be a challenge in many parts of the world.
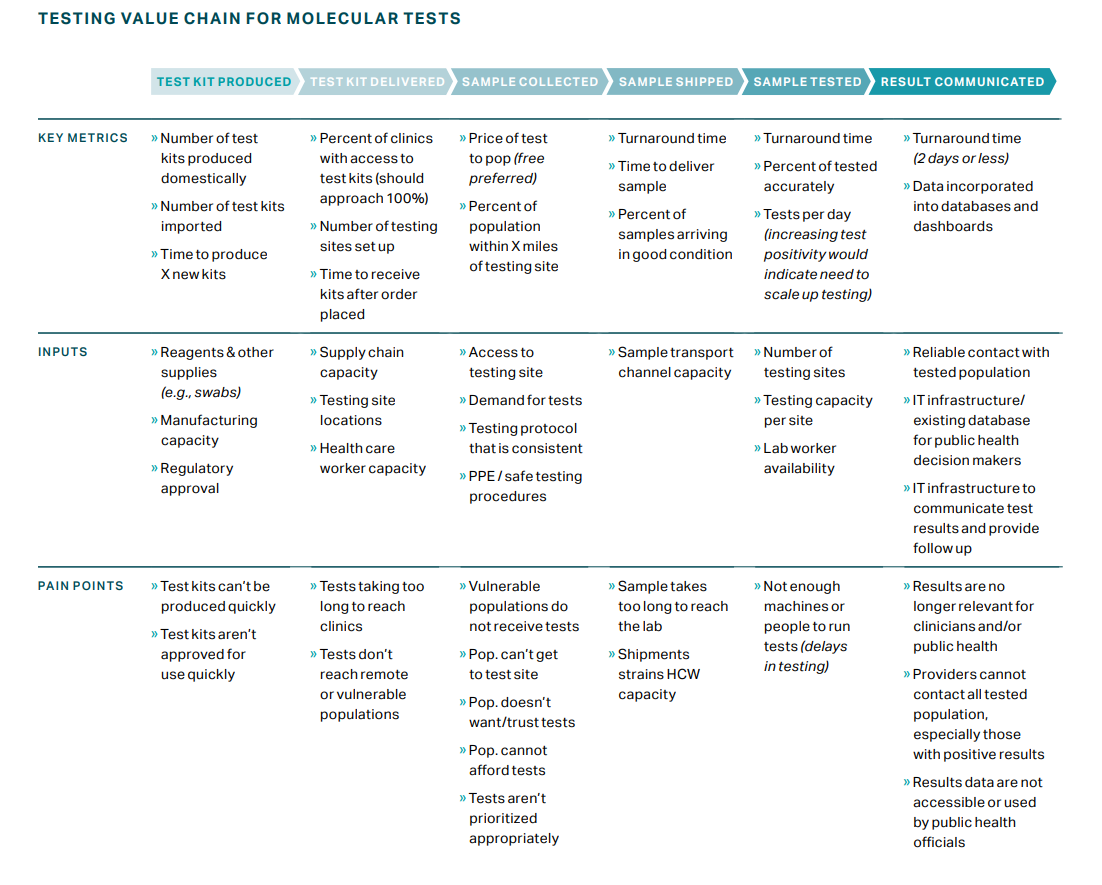
This value chain was designed for molecular tests. If antigen and antibody testing are integrated into a broader testing strategy, the framework may be adapted. Additionally, several problems can occur once a result has been generated that are outside the scope of this framework (e.g., limited contact tracing).
What Do We Know about Surveillance for COVID-19?
Surveillance is a top priority in epidemic management and control. Countries’ responses to emergent health crises such as COVID-19 depend in large part on the strength of the surveillance systems they establish.
In the initial days of a COVID-19 outbreak (or an outbreak of any other virus), health departments often will initiate testing to identify whether a virus is present and when (and where) it arrived. In addition to surveillance, subsequent testing focuses on supporting clinical care to ensure patients with the most severe symptoms get the care they need. Finally, as health systems get the acute phase of the pandemic under control, they can move to the surveillance phase in which they continue to care for sick patients while attempting to define the public health contours of the epidemic. In this phase, effective surveillance systems collect and consolidate data, confirm cases, identify patterns of disease progression and spread, and efficiently inform public health action.
According to the World Health Organization, the objectives of COVID-19 surveillance are to:
- Enable the rapid detection, isolation, and management of suspected and probable cases
- Detect and contain clusters and outbreaks, especially among vulnerable populations
- Identify, follow up with, and quarantine contacts of confirmed COVID-19 cases
- Guide the implementation and adjustment of targeted control measures while enabling safe resumption of economic and social activities
- Evaluate the impact of the pandemic on health care systems and society
- Monitor longer-term epidemiologic trends and the evolution of SARS-CoV-2, and monitor trends in COVID-19 deaths
- Contribute to the understanding of the co-circulation of SARS-CoV-2, influenza, other respiratory viruses, and other pathogens
To rapidly identify and care for individuals who are sick with COVID-19, trace and quarantine their contacts, and monitor disease trends over time, most countries’ health systems need to strengthen their surveillance capacities significantly. Comprehensive national surveillance for COVID-19 will require the adaptation and reinforcement of existing national systems where appropriate and the scale-up of additional surveillance capacities as needed.
Common challenges include:
- Slow paper-based surveillance systems making real-time monitoring impossible
- Sampling from a representative sample of the population
- Integrating data from a variety of different sources (geographic locations, institutions, incompatible information systems) into one system
Health systems can build and support these capacities using digital technologies for contact tracing and other functions. For example, the open-source, web-based health information management system District Health Information Software 2 () and the real-time epidemic-monitoring tool Surveillance, Outbreak Response Management and Analysis System () can both provide more timely and accurate data than traditional paper-based surveillance systems. They can also enable and accelerate data collection, analysis, visualization, and sharing to inform the development of policies for epidemic prevention and control. Surveillance systems should be geographically comprehensive, and surveillance for vulnerable or high-risk populations should be enhanced. Ideally, a country’s surveillance system is one robust, integrated system that can accurately capture representative population health and the outbreak burden through an array of data sources.
Examples of Surveillance Methods and Systems across Different Sites and Contexts
| Surveillance Method | Definition |
|---|---|
Case investigation | Process of confirming that a person with COVID-19 knows their positive status, encouraging self-isolation, providing guidance, and gathering additional information on the person’s symptoms, underlying health conditions, locating information, and workplace role and location. |
Contact tracing | Process of notifying contacts of exposure, addressing questions and concerns, referring for SARS-CoV-2 testing, encouraging self-quarantine, monitoring symptoms, and assessing the contacts’ need for supportive services. |
Community-based surveillance | Systematic detection and reporting of events of public health significance within a community. It is important for identifying cases and trends that might not be captured by the health system, particularly for asymptomatic or mild infections. |
Surveillance at the primary care level | Surveillance in primary care settings via testing at clinics or fixed sites in the community. Regular data reporting to local or national public health authorities is often facilitated through online systems, mobile apps, SMS messages, or telephone. |
Laboratory-based surveillance | Surveillance that relies on the collection of information about bacteria that have been identified by laboratory testing of ill persons. |
Hospital-based surveillance | Process of identifying and notifying national health authorities of probable or confirmed COVID-19 cases admitted to hospitals. |
Sentinel site surveillance | Process of testing people across the community, including those who appear well, to discover unseen transmission. Countries may conduct primary care or hospital-based sentinel surveillance for influenza-like illness, acute respiratory infection, severe acute respiratory infection, pneumonia, and other diseases. |
Mortality and postmortem surveillance | Reporting of deaths from COVID-19 by civil registration and vital statistic systems. Investigation of suspected cases postmortem through medical autopsy can further reveal the true toll of COVID-19. |
Serological surveillance | Population-based surveys of antibody seropositivity and the use of serology in specific settings or populations to estimate the proportion of the population that has been infected with SARS-CoV-2. |
Event-based surveillance | Process of capturing informal and formal information from channels such as online content, radio, and print media to identify potential public health risks. This serves as a complement to other surveillance efforts. |
Telephone hotlines | System for trained staff to triage calls and appropriately refer callers to health care or other services. |
Genomic sequencing and pathogen surveillance | Process of decoding genes to learn more about the virus, which allows scientists to identify SARS-CoV-2 and monitor how it changes over time into new variants, understand how these changes affect the characteristics of the virus, and use this information to better understand how it might impact health. This informs therapeutic and diagnostic accuracy as well as genomic epidemiology. |
Environmental surveillance | Process of testing untreated wastewater, air, surfaces, and other points in the environment to monitor the presence of SARS-CoV-2, detect cases in a community, and monitor circulation of the virus during outbreaks. |
Operational considerations for adapting a contact tracing program to respond to the COVID-19 pandemic in non-US settings. Centers for Disease Control and Prevention website. Updated June 23, 2021. Accessed August 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/global-covid-19/operational-considerations-contact-tracing.html
What is genomic surveillance? Centers for Disease Control and Prevention website. Updated June 17, 2021. Accessed August 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-surveillance.html
World Health Organization (WHO). Public Health Surveillance for COVID-19. Geneva: WHO; 2020. https://apps.who.int/iris/bitstream/handle/10665/337897/WHO-2019-nCoV-SurveillanceGuidance-2020.8-eng.pdf?sequence=1&isAllowed=y
Coronavirus disease (COVID-19): environmental surveillance. World Health Organization website. Published December 2, 2020. Accessed August 12, 2021. https://www.who.int/news-room/q-a-detail/environmental-surveillance#:~:text=early%20warning%20for%20COVID%2D19,hotels%2C%20university%20campuses%20or%20prisons.
Vital Strategies and World Health Organization. Revealing the Toll of COVID-19: A Technical Package for Rapid Mortality Surveillance and Epidemic Response. New York, NY: Vital Strategies; 2020. https://cdn.who.int/media/docs/default-source/documents/ddi/rms_report_v04-(2)3cb3c4d5-d98d-4dcf-b6fc-39bdbfb1a51d.pdf?sfvrsn=4950b624_1&download=true
How Have the Four Countries Performed in Testing and Surveillance This Far?
Testing: Across the four countries studied, officials leveraged existing testing capacity and public-private partnerships. However, limited resources and lab infrastructure, global supply chain challenges, and a reliance on RT-PCR tests made it difficult to ensure an equitable and reliable supply of tests. Although capacities are increasing, as of July 2021, none of the four countries had integrated rapid diagnostic tests fully into its testing strategy.
Surveillance: The countries have improved data management and surveillance capacity rapidly by training health workers and increasing resources for laboratories, but the disease burden continues to be underdetected. Decentralizing surveillance to enable swifter implementation of targeted public health measures at the subnational level remains a challenge. The need for increased genomic surveillance, postmortem surveillance, and seroprevalence studies persists.
Coordination: The countries have prioritized national-level coordination across sectors and stakeholders. Each country has established or leveraged robust partnerships with nongovernmental organizations, academics, and other global institutions. There is potential to improve coordination at the regional level and to better regulate private actors involved in pandemic response.
Moving forward, there are several actions that countries, regional bodies, and global partners can take to manage COVID-19 and be better prepared for the next major disease outbreak. These include:
- Continue to invest in integrated, user-friendly data systems.
- Establish regional mechanisms for disease surveillance and validation of test results.
- Strengthen regional manufacturing capacity.
- Build effective alert-management systems.
- Improve supply chain management, including via pooled procurement at a regional level.
- Invest in health worker capacity for public health actions such as contact tracing.
- Strengthen partnerships with the private sector and clarify its role in pandemic response.
- Ensure data access and availability for reporting and improve ability to transmit data between and across multiple levels of the health care system.
We describe these measures further in the .
OUR PARTNERS
This research was conducted by the Makerere University School of Public Health, in partnership with the University of Kinshasa in the Democratic Republic of the Congo, Université Cheikh Anta Diop in Dakar, Senegal, and University of Ibadan in Nigeria. The four countries in this study were selected for the variability in their COVID-19 responses and outcomes, their experience in managing past epidemics of global concern, the strong existing partnerships between in-country research institutions and the countries’ ministries of health to facilitate access to data and enable the translation of findings to action, and the representation of Francophone and Anglophone countries to enhance South-to-South collaboration.