Key Points
To effectively reach communities, Bangladesh took initiative with adapting Integrated Management of Childhood Illness (IMCI) to their country context, and even adapted it to the community level before global community-level guidelines were developed.
Immunization programs (including PCV, Hib vaccine, and measles first dose vaccine), prevention efforts (ITN and IRS for malaria), and treatment (ORT and nutrition programs) were key to Bangladesh’s reduction of U5MR.
Bangladesh also focused on reducing neonatal mortality, through improving antenatal care and access to skilled birth attendants.
Diagnosis and Treatment of Diarrhea, Pneumonia, and Malnutrition
Integrated Management of Childhood Illness
The Integrated Management of Childhood Illness (IMCI) strategy was developed in 1995 by the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) to improve the well-being of the whole child. The integrated approach includes preventive and curative care and focuses on improvements in three main areas: case management of health care staff, overall health systems, and family and community health practices.
It focuses on improving the ability of health care providers to diagnose and treat the most common fatal conditions among children in low-income countries, including lower respiratory infections, diarrhea, and malaria.
Bangladesh quickly sought to implement the new strategy. Diarrhea and lower respiratory infections, such as pneumonia, were the two leading causes of mortality among children under the age of five (under-five mortality or U5M). By 2000, these two conditions alone accounted for 31 percent of all U5M deaths in Bangladesh, with lower respiratory infections responsible for 24 percent and diarrhea for 7 percent.
The Control of Diarrhoeal Diseases program and the Acute Respiratory Infections initiative already addressed diarrhea and lower respiratory infections in Bangladesh, but the IMCI strategy promised a more comprehensive approach informed by the latest international findings on how to identify and address these common yet dangerous illnesses.
Preparations for implementing IMCI in health facilities (referred to as facility-based IMCI, or FB-IMCI) in Bangladesh began in 1998, with the establishment of a national steering committee under the leadership of the deputy program manager of the Control of Diarrhoeal Diseases program.

Bangladesh’s version of IMCI was designed to reflect the country’s unique U5M challenges. It targeted not only respiratory infections and diarrhea, but also malnutrition – a longtime concern for a country that had suffered a devastating famine less than a quarter-century earlier. Bangladesh adapted WHO’s guidelines for IMCI and integrated nutrition services into IMCI visits, including growth monitoring and counseling on feeding practices.
Pneumonia, diarrhea, and malnutrition were the primary targets of the IMCI program, but the program included others as well. Malaria was not a major cause of childhood mortality in Bangladesh, but because the country had malaria-endemic regions, the disease was added to the country’s IMCI portfolio. Further details on malaria diagnosis, treatment, and prevention may be found in the malaria sub-section that follows.
In 2001, with financial and technical support from WHO and UNICEF – and technical support from the Bangladesh NGO International Centre for Diarrhoeal Disease Research (ICDDR,B), implementation of FB-IMCI in Bangladesh began with small-scale testing as part of a multicountry evaluation of the IMCI strategy in five countries – Uganda, Brazil, Tanzania, Peru, and Bangladesh.
From 2002 to 2004, researchers conducted surveys at six-month intervals. They found that 94 percent of health workers in facilities implementing the FB-IMCI strategy had received training on the proper protocols.
Even more promisingly, the surveys showed an increase in the quality of care and usage rates at the sites that had implemented FB-IMCI. On a scale of 0 to 100, the FB-IMCI facilities scored an average of 54 for correct treatment of childhood illnesses, compared with an average score of 9 in the control group. Use of FB-IMCI facilities also increased, from 0.6 visits per child per year in 2002 to 1.9 visits per child per year in 2004.
Bangladesh conducted a cost-effectiveness study to identify the recurrent cost implications of adopting and scaling up IMCI, and findings showed that the IMCI strategy could save the country approximately US$4 million on costs of medicines. The savings are based on recommended medication practices for IMCI compared with standards of practice for health care workers outside of IMCI settings and takes into account the additional costs required for personnel needed to implement IMCI.
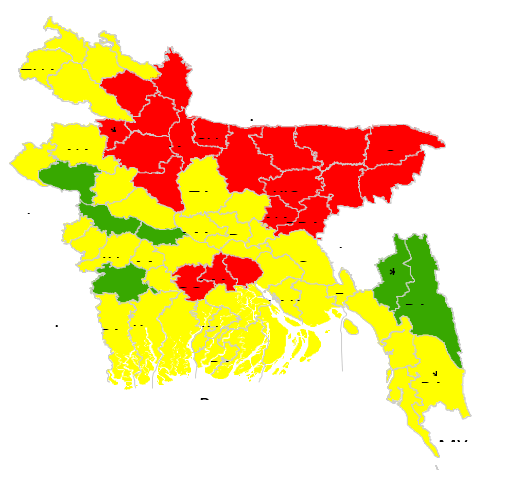
Based on these results, the national steering committee developed a plan for the phased national scale-up of FB-IMCI across Bangladesh. According to informants, financial limitations prevented the government from introducing it across the entire country simultaneously, so the rollout began in districts with the highest U5M rates in December 2004 (shaded red in above figure), followed by districts with moderate U5M rates (shaded yellow), and then in districts with the lowest rates (shaded green).
When Bangladesh decided to implement FB-IMCI, a community-based IMCI strategy (CB-IMCI) had not yet been developed by WHO and UNICEF. Informants mentioned, however, that Bangladesh decided on its own to implement the IMCI strategy at the community level, reflecting the needs of a predominantly rural country in which a large proportion of the population lacked ready access to health facilities and trained medical personnel.
In 2002, a year after the FB-IMCI multicountry evaluation began, the government began planning for a CB-IMCI initiative that could build upon existing CHW programs. Under the leadership of the same national working group that had developed Bangladesh’s FB-IMCI blueprint, the government and its partners adapted FB-IMCI protocols to community-based settings, drawing upon guidelines that had already been put in place for the Control of Diarrhoeal Diseases program and the Acute Respiratory Infections initiative.
Like FB-IMCI, Bangladesh’s CB-IMCI program focused on diarrhea, respiratory infections, malnutrition, and malaria. Reflecting the results from the pilot of FB-IMCI, which showed that drowning was a major cause of death, Bangladesh adapted the program to include this non-medical cause of U5M in CB-IMCI. It took this step based on evidence that drowning was, in fact, one of the leading causes of death of children in Bangladesh during the 2000s.
In 2003, Bangladesh began a pilot of the CB-IMCI program, which revealed that the country needed an additional 2,700 to 4,000 community health workers to implement it nationally, which would cost between US$2.7 million and US$4 million – a price the government was willing to pay.
The following year, WHO adapted Bangladesh’s emerging CB-IMCI model for global use. In 2005, Bangladesh turned its model into a reality, commencing a phased rollout of CB-IMCI in 15 subdistricts with high rates of U5M.
As Bangladesh scaled up CB-IMCI, the government began exploring the feasibility of engaging “village doctors” – traditional healers in rural areas far removed from formal health facilities – to enhance the reach and public acceptance of the new program, given their popularity as the first point of care for children under five.
In 2005, Bangladesh conducted two-day trainings on the CB-IMCI protocol for 144 village doctors, followed by a two-year period in which they were monitored for their implementation of the protocol.
A study on CB-IMCI found that village doctors could adequately implement the CB-IMCI protocol, as demonstrated by their understanding of the proper methods for assessing and managing pneumonia and diarrhea up to two years after training.
For example, their knowledge of the correct management of severe pneumonia and diarrhea increased from 62 percent and 65 percent, respectively, to 84 percent and 82 percent after the training, and remained relatively high at the end of the two-year interval.
Despite these positive findings, the absence of standardized criteria for becoming a village doctor – and the inability to ensure consistent competency among village doctors nationally – resulted in a government decision to limit their CB-IMCI role to one of counseling and referral. With or without the active participation of the village doctors, CB-IMCI (and FB-IMCI) are firmly established in Bangladesh – despite inconsistent results.
Since 2009, FB-IMCI has been incorporated in trained physicians’ undergraduate-training curriculum, yet a 2014 study showed low IMCI readiness among the country’s health facilities – only half had IMCI guidelines on hand, or even one health care provider who had ever received in-service refresher training in the program.
Despite growing pains, the future of FB-IMCI seems secure. When UNICEF and WHO ended their financial support for FB-IMCI in Bangladesh in 2015, the national government increased its budget allocation for IMCI to cover the loss of donor funding and ensure sustainability of the program. As of 2016, data from the Directorate General of Health Services (DGHS) showed that FB-IMCI had nearly achieved implementation at a national scale, reaching 98 percent of all subdistricts.
CB-IMCI faced more challenges in reaching national scale; data from the Ministry of Health and Family Welfare (MOHFW) showed that as of 2016, CB-IMCI had been rolled out in only about half of all subdistricts (249/490) in Bangladesh. However, informants said this figure did not include NGO coverage, and noted that CB-IMCI was implemented in all sub-districts in Bangladesh.
The following sections focus on specific interventions to address the primary medical causes of U5M in Bangladesh targeted by FB-IMCI and CB-IMCI – pneumonia, diarrhea, malnutrition, and malaria – before turning to other significant causes, such as HIV, meningococcal meningitis, and neonatal complications.
Diarrhea: Oral Rehydration Therapy and Scaling Up Zinc for Young Children
The use of oral rehydration therapy (ORT) to treat diarrhea in children was discovered in Bangladesh in the late 1960s, through research led by the Pakistan – Southeast Asia Treaty Organization’s cholera research laboratory (later known as the International Centre for Diarrhoeal Disease Research, Bangladesh, or ICDDR,B).
ORT was well established in Bangladesh by the 1990s, but another proven treatment for diarrhea – zinc supplementation – gained only limited acceptance. This low acceptance was partly due to concerns that promoting zinc for diarrhea treatment could impede the momentum of the ORT campaign.
A study conducted in Bangladesh from 1998 to 2000 provided evidence that promotion of zinc did not result in reduced ORT usage. Other ICDDR,B studies showed that zinc effectively decreased the severity and duration of diarrhea.,, As a result, Bangladesh considered introducing a national zinc program.
The Scaling Up Zinc for Young Children (SUZY) project began in 2003 with small-scale testing to improve awareness of zinc as an important childhood diarrhea treatment. By the end of 2006, more than 5 million zinc tablet packs had been sold across the country, more than the expected demand of 3 million.
By 2007, approximately 95 percent of Bangladeshi mothers of children under five in semi-urban and urban areas were aware of zinc’s usefulness in treating diarrhea in young children. In rural areas, awareness was lower at 50 percent. Though public awareness was strong overall, actual use of zinc to treat diarrhea was low; only 23 percent of diarrhea cases were treated with zinc, with coverage reaching similar levels in both rural and urban areas.
The government decided to change zinc from a prescription drug to an over-the-counter medicine in 2008, which opened up the sale of zinc to retail shops. Despite the increased distribution, however, zinc still has not achieved full acceptance. A 2012 study found that parents and other caregivers remained reluctant to treat diarrheal cases with zinc, because its benefits were not as obvious to them as the proven merits of ORT.
According to the Demographic and Health Survey (DHS), the proportion of children with diarrhea who received zinc supplements increased from 23 percent in 2007 to 49 percent in 2014., The proportion of children with diarrhea who received ORT (either oral rehydration salts - ORS - or recommended homemade fluids - RHF) increased from 72 percent in 2000 to 83 percent by 2014.
Both ORT and Zinc treatment were integrated into IMCI protocols for diarrhea.
Malnutrition
Bangladesh Integrated Nutrition Program
In 1995, Bangladesh sought new ways to address high levels of malnutrition among children and women of childbearing age. As a result, the government introduced its first large-scale nutrition initiative, the Bangladesh Integrated Nutrition Program (BINP), implemented in 61 of 490 sub-districts. The main focus of the program was on nutrition activities at the community level.
Bangladesh partnered with NGOs who were tasked with implementing the Bangladesh Integrated Nutrition Program. Program headquarters were staffed by personnel seconded from the MOHFW personnel, but the program itself was administratively separate from the ministry.
Under the BINP, levels of participation were high in growth-monitoring sessions (75 percent to 95 percent of children) and nutrition education sessions (66 percent of mothers). Data on supplementary feeding for children, however, showed low coverage, with only 21 percent of eligible children receiving it.
An evaluation of the BINP found meaningful progress toward reducing malnutrition. From the beginning of the BINP in 1995 until its conclusion in 2002, severe malnutrition dropped from 13 percent to 0.9 percent, and moderate malnutrition declined from 32 percent to 16 percent in targeted districts.
The evaluation also revealed, however, that central-level staff lacked the management skills required to oversee such a large enterprise. The decision to establish the BINP as a separate program was seen at the time as a sound, proven practice, but according to informants, the establishment of the BINP office as a vertical program outside the MOHFW meant that no one at the ministerial level had a sense of responsibility for it.
“In Bangladesh, there were a lot of vertical programs at that point in time,” one informant explained. “The vertical programs were implemented partly because of the success of the immunization program in Bangladesh, which was also implemented as a vertical program . . . everybody wanted to replicate that.”
National Nutrition Program
In 2002, the BINP was reorganized and given a new name – the National Nutrition Program (NNP). The new program adopted many of the same objectives as the BINP, but it placed a greater emphasis on coordination between the central government and the NGOs working in the communities. The NNP also covered a larger area than the BINP, moving into an additional 79 subdistricts before eventually expanding to the rest of the country.
In 2006, the NNP ended when it was integrated with the cross-cutting Health, Nutrition and Population Sector Program (HNPSP) under the Sector-Wide Approach (SWAp) initiative.
The prevalence of stunting decreased from 51 percent in 2004 to 41 percent in 2011, following the implementation of the NNP and subsequent nutrition activities through the HNPSP. The proportion of underweight children also declined – from 43 percent to 36 percent during the same period, but wasting (low weight in proportion to height) increased slightly, from 12 percent in 2004 to 16 percent in 2011.
Taking a longer view – from 1996 to 2014 – the positive trends are clearer still. During this period, the prevalence of stunting decreased from 60 percent to 36 percent, the proportion of underweight children from 52 percent to 33 percent, and the prevalence of wasting from 21 percent to 14 percent.
Despite clear progress, rates of malnutrition remain troublingly high. “The dietary diversity of the children has not been improved in Bangladesh, [nor has] the quality of diet,” said one informant. “The diet is mainly carbohydrate [and] density of micronutrients is poor. The macronutrient contents of those diets are also poor.”
A 2018 study underscored ongoing challenges, finding that 35 percent of Bangladesh’s population remained food insecure.

For more information on the continuing problem of food insecurity, see the article in this narrative.
Vitamin A Supplementation
One subcategory of malnutrition is vitamin A deficiency, which puts children at greater risk of death due to common illnesses such as respiratory disease, measles, and diarrhea. Several studies carried out in different countries have demonstrated that vitamin A supplementation (twice a year with high-dose capsules) significantly reduces mortality among children 6-59 months of age. WHO and UNICEF have recommended introducing vitamin A supplementation programs in all countries with high infant and child mortality rates, including Bangladesh.
Bangladesh has used several strategies to increase distribution of vitamin A, both before and during the study period. In 1973, the national government began a free high-dose vitamin A capsule distribution program twice a year for all children from birth to six years, with financial support from UNICEF. By 1989, however, program coverage remained low – especially in rural areas, where only 35 percent of children had received the capsules.
In the mid-1990s, Bangladesh integrated vitamin A supplementation into its facility-based immunization program, changing the dosing for children ages 0 to 11 months to coincide with vaccination schedules. Children ages 12 months to six years continued to be targeted through home visits and twice-annual dosing, with coverage levels ranging from 60 percent to 67 percent during the study period.,
Malaria
As a whole, Bangladesh has relatively low rates of malaria incidence and mortality, but it is endemic in 13 northeast and southeast districts, accounting for 98 percent of all malaria cases in Bangladesh.
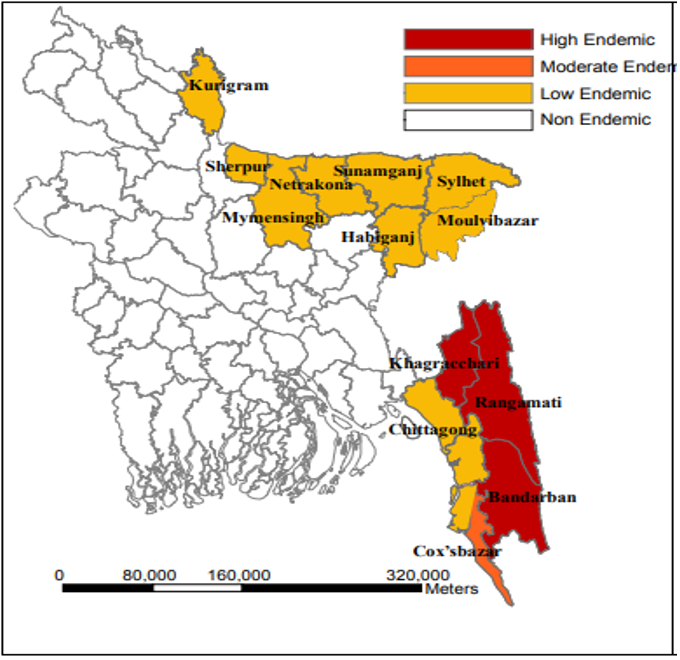
According to informants, the modest levels of malaria incidence in Bangladesh during the 1980s led to a de-emphasis of malaria programming, resulting in cuts to funding for indoor residual spraying (IRS), which had been the mainstay of antimalaria efforts. As a result, the malaria incidence rate increased from 6 cases per 1,000 people in 1990 to 19 in 1994 – a 217 percent increase.
Alarmed, MOHFW officials began advocating for a new national malaria strategy. In 1994, Bangladesh adopted the Revised Malaria Control Strategy, with a renewed focus on reducing malaria morbidity and mortality through prevention, early diagnosis, and prompt treatment.
Indoor Residual Spraying
Between 1960 and 1977, Bangladesh’s malaria program implemented IRS as a key component of its vector-control strategy in endemic districts, using WHO-supplied dichloro-diphenyl-trichloroethane (DDT). By 1994, with the introduction of the Revised Malaria Control Strategies program, Bangladesh moved away from DDT because of its environmental effects. By 1998, the chemical was banned in Bangladesh.
In 2006, WHO released a position statement recommending the scale-up of IRS as a means to achieving the malaria-related Millennium Development Goals by 2015. Because of the ban on DDT, however, Bangladesh did not reintroduce spraying until 2013, when it began using pyrethroids. Instead of the blanket-spraying approach used before 1998, Bangladesh focused its IRS program on “hotspots” – targeting any village segment (known as a para) with more than two or three malaria cases per year.
According to informants, the adoption of a hotspot approach was informed by research showing that it was more feasible and cost-effective than blanket spraying.
Insecticide-Treated Nets
In 1998, BRAC (originally the Bangladesh Rehabilitation Assistance Committee, now known only by its initials) began distributing insecticide-treated nets (ITNs) in the high-endemic southeastern districts of Bandarban, Khagrachari, and Rangamati as part of the worldwide Roll Back Malaria initiative, with technical support from WHO.
The BRAC program ended in 2004, and the government began preparations in 1995 for an expanded malaria program to provide long-lasting insecticidal nets (LLINs) to all households in three high-endemic districts, and to 80 percent of households in the other ten endemic districts – all of which were either in the southeast or the far northeast of Bangladesh. In 2006, the Global Fund to Fight AIDS, Tuberculosis and Malaria approved a grant for the proposed program.
Bangladesh began distributing LLINs in 2007. A consortium including BRAC and 20 other NGOs with experience in the malaria-endemic districts was established to guide the distribution program, with oversight from the government. WHO personnel trained CHWs from BRAC’s Shasthya Shebika community health worker program and other NGOs in community engagement techniques and the importance of LLIN use.
Guidelines for the distribution of LLINs in Bangladesh reflected WHO recommendations to issue a minimum of one net for every two individuals in a household, and to replace the nets every three years (based on evidence showing that the average LLIN was effective for three to five years). The nets were distributed for free in schools and other community spaces.
A study conducted between 2008 and 2011 found that the proportion of households with at least one LLIN in the northeast increased from 22 percent in 2008 (one year after the program began) to 62 percent in 2011. In the southeast, the proportion rose from 60 percent in 2008 to 67 percent in 2011.
The same study found by 2011, 87 percent of children under five in the northeast slept under an LLIN or other ITN and 92 percent in the southeast. Among pregnant women, 83 percent in the northeast slept under an LLIN or other ITN and 91 percent in the southeast.
Research conducted between 2008 and 2012 also found a reduction in malaria incidence and prevalence associated with ITNs in Bangladesh. Research showed one LLIN (or other ITN) for every 2.6 people in the high-endemic districts as of 2012, falling just short of the target of two people per net.
The study also found that in districts that met the target, severe malaria declined by 25 percent, and overall malaria cases dropped by 21 percent. Malaria-associated mortality among all age groups fell by 76 percent, compared with baseline data.
Rapid Diagnostic Tests and Artemisinin-Based Combination Therapy
Before 2007, the FB-IMCI program in Bangladesh used facility-based microscopy to diagnose malaria. That year, with financial and technical support from the Global Fund, the government introduced rapid diagnostic testing (RDT) for malaria diagnosis in endemic areas – three years before WHO issued its recommendation for such a policy.
An informant explained that this early adoption of RDTs was mainly based on ease of use in the field, at least compared with microscopy: “Where you’re going to have a microscope, you have to have it clean. Keeping it outside, you cannot do it. But RDT is different.”
Bangladesh rolled out RDTs nationally without first conducting small-scale piloting. When asked why, an informant explained, “I would say it was rolled out because the money from Global Fund came and it was well established globally that this is well accepted. So it didn’t have to be piloted.”
At the beginning of the FB-IMCI program in Bangladesh, global and country-led surveillance studies had begun to identify increasing resistance to chloroquine, the country’s frontline malaria treatment.
Acting on WHO recommendations, Bangladesh switched from chloroquine to artemisinin-based combination therapy (ACT) in 2004. But implementation of ACT was delayed by three years because of financial constraints. By 2007, right at the time of the RDT rollout, the Global Fund began providing support to Bangladesh for the scale-up of malaria interventions.
With this funding, Bangladesh introduced ACT nationally – administering rapid diagnostic tests for all fever cases and treating the malaria-positive ones with ACT (an approach known as “test-to-treat”), at no cost to the patient.
In 2015, Bangladesh kicked its malaria efforts into higher gear, adopting a pre-elimination strategy that involved active testing, diagnosis, treatment, and tracking of all malaria cases in an aim to achieve a malaria-free Bangladesh by 2030.
With support from the Global Fund, RDTs were rolled out nationally, not only in the endemic districts. But to keep costs down, only those fever cases with no other possible causes were tested with RDTs. “In [high-endemic areas] I would say we go for any fever case,” said an informant. “But in the plain land [low-endemic areas], you have to exclude other causes [so that] we won’t waste our RDTs.”
Between 2007 and 2015, more than 2 million RDTs had been conducted in Bangladesh. Feverish children under five taking ACT remained low at 1 percent in 2011 and 4 percent in 2014, suggesting an increase in accurate malaria diagnosis and appropriate treatment, and continued low levels of endemicity.
In 2008, a year after the RDT program began, malaria incidence was 6.2 cases per 1,000 people. By 2012, after the introduction of RDTs and increased distribution of bed nets, the number of cases fell to 2.1. Malaria incidence had declined by 65 percent, severe malaria incidence by 79 percent, and malaria-associated mortality by 91 percent.
According to IHME estimates, U5M attributable to malaria in Bangladesh declined by 69 percent between 1990 and 2016, falling from 0.02 deaths per 100,000 live births to 0.008. Given the low rates of malaria deaths nationally, the reduction in U5M was not a significant driver of overall child mortality reductions in Bangladesh. However, prevention and treatment interventions contributed to meaningful declines in malaria deaths among children in endemic districts. Subnational analysis of the impact of these interventions on child mortality was not available for this study.
Immunization
In 2000, at the beginning of the study period, Bangladesh’s immunization program provided six vaccines for children – for polio; diphtheria, pertussis, and tetanus (DPT); tuberculosis Bacillus Calmette-Guérin (BCG); and measles. This lineup had gone essentially unchanged since 1979, when Bangladesh launched its Expanded Programme on Immunization (EPI). Initially, vaccines were given only at national, divisional, and district-level hospitals. Due in part to the limited number of delivery points, vaccine coverage remained low – less than 2 percent in 1984.
In 1985, Bangladesh committed to achieving universal child immunization by 1990, a benchmark recommended by WHO. To reach this goal, the government set up an interagency coordinating committee in 1985 to oversee all EPI activities, with members representing government ministries, NGOs, and donors.
The government established eight immunization centers within every ward across the country and promoted the importance of immunizations for children in their first year of life, and for women of childbearing age.
As a result of these efforts, vaccination coverage improved throughout Bangladesh – especially for BCG, which reached 85 percent coverage by 1993. Yet major gaps still remained, with DHS data showing that by 1993 only 59 percent of Bangladeshi children ages 12 to 23 months had received all eight of the basic immunization doses covered under the EPI (three doses each of DPT vaccine and polio vaccine, plus single doses of measles vaccine and BCG).
Between 2000 and 2015, Bangladesh introduced four new vaccines to its national immunization program, including hepatitis B vaccine, Haemophilus influenza b (Hib) vaccine, rubella vaccine, and pneumococcal conjugate vaccine (PCV). These vaccines were introduced in response to WHO and Gavi recommendations, and in response to the available national data on disease burden.
In 2000, 60 percent of children ages 12 to 23 months had received all eight basic immunization doses, increasing to 84 percent in 2014. Economic inequities remained, however. Only 50 percent of children in the poorest wealth quintile had received all eight vaccines in 2000, compared with 69 percent of children in the wealthiest quintile; by 2014, coverage increased to 75 percent and 92 percent, respectively.,
Measles Vaccine
Measles vaccination coverage was 71 percent in 2000, still well below the level needed to stop transmission. Consequently, Bangladesh continued to experience measles outbreaks. In 2003, the country established an outbreak surveillance system, using the system it had previously used to reduce polio.
In 2004, Bangladesh introduced a Measles Control Action Plan, with the goal of increasing coverage to 90 percent by 2010 – the minimum level needed to ensure sufficient herd immunity. To achieve this, the government introduced a supplementary immunization activity (SIA) program – catch-up vaccinations intended to reach children who had missed their recommended dosages the first time around.
Bangladesh conducted its first measles SIA in 2005–2006, an endeavor that included preliminary cold chain assessment and strengthening with support from UNICEF to ensure sufficient capacity for the number of measles vaccines that the country was about to distribute; the aim was to vaccinate more than 35 million children between the ages of nine months and ten years. The decision to set the upper age limit at ten years was based on data showing that 89 percent of all serologically confirmed cases of measles were among children younger than ten.
The SIA exceeded its coverage goal, with 36 million children receiving the measles vaccine. Following the SIA, the number of measles cases in Bangladesh fell sharply, from 25,934 cases during the 2005 outbreak to 6,192 in 2006, and 718 in 2009.
The success of the 2005–2006 SIA convinced the government to conduct another SIA in 2010. This time, the upper age limit was five years, to ensure that all children born between 2006 and 2010 received the vaccination.
Unlike the earlier SIA, planners decided to forego further strengthening of the vaccine cold chain, with the assumption that the system now had sufficient capacity. This assumption was not entirely accurate, however; an evaluation conducted in 2011 found that in some rural areas, the numbers of refrigerators and cold boxes had been inadequate to meet the expanded demands of the 2010 campaign.
While this SIA achieved 100 percent coverage, vaccinating 18.1 million children, Bangladesh’s surveillance data soon identified a sharp increase in national measles incidence – from 5.3 cases per 1 million people in 2010 to 37.4 in 2011. Even more disturbingly, 86 percent of the laboratory-confirmed cases were among children and adolescents younger than 15.
In 2012, with co-funding support from Gavi, Bangladesh introduced a children’s measles vaccine that provided children ages 15 to 18 months with a second dosage. It also protected from rubella, which still had a high incidence rate among children and young teenagers.
Two years later, another national SIA provided the measles-rubella vaccine to 53 million children between the ages of nine months and 15 years, achieving 100 percent coverage.
According to DHS data, national measles vaccination coverage had increased to 86 percent in 2014, but coverage still remained below the 90 percent threshold the government had hoped to meet four years earlier.
IHME found that measles deaths among children under five in Bangladesh had declined from 81 per 100,000 children in 1990 to 47 in 2000 – and that this rate had plunged further to 37 in 2005, following the first SIA, and then to 1 per 100,000 children in 2016.
A separate 2017 report confirmed that overall measles incidence had declined by 82 percent, from 34.2 per 1 million people in 2000 to 6.1 in 2016.
In 2017, after the study period ended and Gavi co-funding for the measles-rubella vaccine ended, the government began procuring the vaccine on its own. The need for measles vaccine coverage still remained, however, even with the country’s impressive progress. As of 2019, the measles vaccine coverage rate was still below 95 percent, and informants said measles outbreaks remained a threat.
Haemophilus Influenzae Type B Vaccine
From 1987 to 1994, Bangladesh conducted a laboratory-based study of diagnosed bacterial meningitis at Dhaka Shishu Hospital, a national pediatric hospital. It found that 47 percent of cases had been caused by Haemophilus influenzae, and that 98 percent of those cases were caused by the disease’s type b strains (Hib).
The study provided evidence of an increase in Haemophilus influenzae meningitis in Bangladesh and strongly recommended introduction of the Hib vaccine, which was used in about half of all high-income countries at that time but was considered too costly for many lower- and middle-income countries such as Bangladesh.
When Gavi was established in 2000 for the exact purpose of eliminating such gaps between vaccine demand and affordability, the organization soon set its sights on Hib, seeking to expand the Hib vaccine’s global coverage through its New and Underused Vaccines Support program. Within five years, Gavi offered a global US$37 million grant to improve Hib uptake, and WHO recommended that all countries include Hib in their immunization programs.
According to informants, the availability of Gavi funding prompted Bangladesh to consider the introduction of the Hib vaccine. In June 2006, the government hosted an interagency workshop to assess the country’s Hib disease burden and determine the technical, programmatic, and financial implications of rolling out the Hib vaccine in Bangladesh.
At the end of the workshop, which included participants from the World Bank, WHO, and UNICEF, the government decided to introduce the Hib vaccine as part of a new pentavalent vaccine that also targeted DPT and hepatitis B. A major factor in the decision had been the body of evidence showing the high levels of effectiveness that the Hib vaccine had attained in other countries; between 90 percent and 99 percent of children receiving three doses had developed antibodies.
The introduction of the pentavalent vaccine was planned for 2008. It was co-financed by Gavi, with Bangladesh responsible for only 20 percent of the cost. However, a delay in receipt of the Gavi vaccine introduction grant hindered the development and printing of training and communication materials; meanwhile, the government learned that many health facilities still stocked hepatitis B vaccines that would go to waste during the introduction of the pentavalent vaccine.
As a result, the introduction of the new pentavalent vaccine was rescheduled for 2009. To ensure that all vaccine-delivery personnel received the necessary training, the rollout began with a small-scale launch in Khulna District in January 2009 and was rolled out across the country by July 2009.
A post-rollout evaluation in March 2012 showed disparities in the quality of implementation between rural and urban areas – in this case, rural residents and disadvantaged city dwellers benefited more than urban residents due to better-quality implementation. As was typical for EPI programs, the MOHFW took the lead outside the main metropolitan areas. Community engagement and public acceptance were high, as was the quality of logistical and delivery practices ranging from cold chain management to injection techniques.
In Dhaka and other urban areas, NGOs were primarily responsible for implementation. Staff turnover was high, especially in the capital, and vacancies resulted in inadequate vaccine delivery and supervision. In addition, the evaluation found that many parents and other caregivers were unaware of the dates and locations of immunization sessions because of poor community engagement.
Pneumococcal Conjugate Vaccine
Gavi began supporting the introduction of PCV in 2000 in lower- and middle-income countries to treat pneumonia and other pneumococcal diseases. Bangladesh soon began advocating for funds to introduce PCV into its immunization program.
By 2006, WHO recommended the introduction of PCV in all routine vaccination programs. The following year, Bangladesh conducted a study to determine the appropriateness of existing PCV types for the country’s specific serotype profile. This study showed that the PCV-10 and PCV-13 serotypes accounted for 46 percent and 50 percent of cases, respectively. However, given the similar effectiveness of the two vaccine types, and the heightened cold chain requirements of PCV-13, Bangladesh opted for PCV-10.
The MOHFW decided in 2013 to introduce PCV; by 2015, PCV was launched as part of its routine immunization program, becoming the second country in South Asia to do so, after Pakistan. Gavi provided most of the funding for the rollout, with Bangladesh picking up 20 percent of the cost. Bangladesh distributed PCV countrywide, without any small-scale testing.
While discussing the decision to roll out the program nationally, one informant explained that Bangladesh had a history of openness to vaccines, describing the country as a “pro-vaccine” country, adding that “if you come to a national immunization day in this country, you will see what a long queue [there is]. To get the vaccine, mothers are coming and waiting for hours on the queue to get the vaccine. There is hardly any country like this. So, Bangladesh has got magic, it’s a kind of magic. . . . Our EPI is unbelievable.”
Challenges still remained, however. Seven months after the PCV rollout, an evaluation found that high turnover had resulted in staff administering the vaccine without adequate training or supervision. This problem was mainly limited to Dhaka, where NGOs had primary implementation authority, and reflected the ongoing difficulty of ensuring adequate supervision of NGO health programs.
In response, the government organized follow-up trainings for vaccinators, increased the number of vaccination supervisors, and developed supervision plans to ensure documentation of supervisory visits and adequate follow-up sessions.
Despite initial glitches, the PCV rollout effort was successful. According to WHO, in 2015 PCV coverage in Bangladesh was 48 percent, with introduction partway through the year – by 2016, coverage had increased to 97 percent.
Rotavirus Vaccine
In response to findings of a high burden of rotavirus-related diarrhea, Bangladesh submitted a proposal in 2016 for Gavi support to introduce a rotavirus vaccine in 2018. Cold chain capacity improvements delayed the planned introduction of the rotavirus vaccine until 2019.
However, a global scarcity of the Rotarix vaccine (which Bangladesh selected over Rotateq due to Rotarix’s comparable effectiveness and less onerous cold chain requirements) further postponed the introduction until 2020.
Estimating impact of vaccinations
Overall increases in vaccination coverage made meaningful contributions to declines in child mortality in Bangladesh. A statistical analysis conducted by the Institute for Health Metrics and Evaluation found that 13.9 percent of the reduction in under-five deaths from 2000 to 2017 can be attributed to vaccines, including the Hib vaccine (5.9 percent reduction), PCV (3.8 percent), DTP3 (2.9 percent), and measles first dose vaccine (1.3 percent) .
Neonatal Mortality
As noted in the introduction to this article, the reduction in Bangladesh’s neonatal mortality rate (NMR, with “neonatal” defined as the first 28 days of life) during the study period was less marked than the decline in overall U5M. However, the reduction in neonatal mortality accounted for 40 percent of the overall reduction in under-five mortality during this period.
Bangladesh’s NMR declined from 42 deaths per 1,000 live births in 2000 to 23 in 2015 – a 45 percent improvement.
As with U5M overall, the decline in NMR occurred across wealth quintiles, but with a steeper decline at the higher end of the income scale. Among the poorest quintile, neonatal mortality declined between 69 deaths per 1,000 live births in 1993 to 41 in 2014 (a 41 percent drop), whereas among the wealthiest 20 percent NMR fell from 42 to 20 during the same time period (a 52 percent decline).
Neonatal mortality decreased in all regions but occurred more slowly in the northwest, northeast, and southeast – in parallel with reductions in U5M.,
The government sought to incorporate measures to reduce NMR into its FB-IMCI programming, setting the minimum age limit for FB-IMCI at 24 hours, rather than seven days as recommended by WHO and UNICEF. According to one informant: “Initially, WHO said IMCI starts after seven days, but we did some changes because the mortality rate was significantly contributed [to] by neonatal mortality in this country, so we wanted to give more coverage to neonatal care.” Eventually the FB-IMCI program was adapted to add interventions targeting newborn illnesses, including sepsis, feeding counseling, and jaundice. The program was renamed Integrated Management of Newborn and Childhood Illnesses (IMNCI) in 2017.
By contrast, the CB-IMCI program’s lower age limit was set at one month, excluding the neonatal period altogether. According to informants, this limit was set because neonatal conditions required higher levels of care that could not be addressed at the community level.
Access to Antenatal Care and Skilled Birth Attendants
Throughout the 1990s, antenatal care (ANC) attendance remained low in Bangladesh, with only 35 percent of pregnant women attending one or more sessions in 1999, and 11 percent attending the WHO-recommended minimum of four sessions. According to informants, these low attendance rates were the result of long-standing cultural preferences for community-based pregnancy and delivery care, often supervised by unskilled practitioners.
Through a variety of CHW programs, some government-led and some supervised by NGOs, Bangladesh was able to achieve high levels of ANC and improved access to skilled birth attendants.
In an effort to improve ANC attendance, access to skilled birth attendants, and postpartum care services, Save the Children launched the Saving Newborn Lives (SNL) initiative in 2000 with funding from the Bill & Melinda Gates Foundation.
Preparations for introducing the SNL initiative in Bangladesh included a participatory design workshop attended by MOHFW personnel, partners such as Save the Children, and research institutions such as ICDDR,B to design and adapt the program to Bangladesh.
A national technical working committee was set up to oversee the SNL initiative, consisting of pediatric experts, child health advocates, public-sector leaders, development partners, international NGOs, and professional societies. The committee worked with the government to advance neonatal health issues and provide technical assistance.
SNL rolled out in 2001 as a collaboration between Save the Children, the MOHFW, ICDDR,B, and Johns Hopkins University. It was introduced initially in three rural Sylhet subdistricts, selected for their relatively higher neonatal, infant, and maternal mortality rates, as well as their high proportion of at-home deliveries.
The purpose of this pilot phase, referred to as the Project for Advancing the Health of Newborns and Mothers (ProjAHNMo I), was to generate evidence that could inform SNL introduction at the national level.
ProjAHNMo I identified women within the communities to be trained as project CHWs. These CHWs then identified pregnant women in the community, who they visited twice during pregnancy (at 12–16 weeks and 32–36 weeks), during delivery, within 24 hours of delivery, and three times within the first week after delivery.
At these visits, the CHWs were required to encourage women to attend ANC sessions. They also provided household-level ANC education and counseling not only for pregnant women, but also for senior female family members, other married women of reproductive age, and adult men within the home.
ProjAHNMo I ended in 2006, and although data on its effect on ANC coverage were not available for the team to review, results showed that overall the community‐based package reduced the neonatal mortality rate by 34 percent.
As discussed in the section, an important element of Bangladesh’s efforts to improve ANC access were community-based skilled birth attendants, deployed in 2003–2004. These workers were trained to provide ANC, perform normal deliveries at home, refer complicated cases to facilities, and provide postpartum care.
Small-scale testing of this program began in 2003 across six districts, with technical and financial support from WHO and UNFPA and technical support from the Obstetrical and Gynaecological Society of Bangladesh.
In 2004, 52 percent of all ANC sessions in the six districts were performed by the community-based skilled birth attendants, suggesting a fairly high level of acceptability. The assessment also found that 91 percent of all women who used these services were either “fully satisfied” or “fairly satisfied” with the sessions.
In addition, the retention of selected skills (including blood pressure measurements and ANC physical examinations) among community-based skilled birth attendants was high, with an average score of 75 out of a possible 100.
The promising outcomes of the testing phase led the government of Bangladesh to scale up the community-based skilled birth attendant program using a phased approach, adding an average of ten districts per year with support from WHO and UNFPA.
In 2009, Bangladesh introduced another cadre of CHWs, community health care providers, to further expand antenatal and postnatal care delivery at the community level, and to provide CB-IMCI in community clinics. The aim to have one community health care provider per community clinic was nearly achieved, with approximately 96 percent recruited in the first year.
Shortly after the introduction of community-based skilled birth attendants, 51 percent of women attended at least one ANC visit in 2004. This number increased to 55 percent by 2011 and 64 percent by 2014.,
While skilled birth attendance in Bangladesh increased after the introduction of community-based skilled birth attendants – from 12 percent in 2000 to 32 percent in 2011 and 42 percent in 2014 – fewer than 0.4 percent of all births from 2004 to 2014 were carried out by these skilled attendants. By 2014, the community health care providers carried out only 1.3 percent of ANC sessions.
In addition, the proportion of expectant mothers attending the WHO-recommended four or more ANC sessions remained low: 17 percent in 2004, 26 percent in 2011 (shortly after the introduction of the community health care providers), and 31 percent in 2014.
Facility-based deliveries overall increased from 9.3 percent of births in 2004 to 37.4 percent in 2014, which was largely due to increases in private-sector deliveries rather than the broader use of government facilities such as upgraded family welfare centers.
A statistical analysis conducted by the Institute for Health Metrics and Evaluation (IHME) found that 2.5 percent of the reduction in under-five deaths from 2000 to 2017 can be attributed to the increased use of skilled birth attendants at delivery. In addition, 10 percent of the reduction in under-five deaths was attributed to a decrease in incidence of low birth weight and short gestational age, which were likely to have been at least partly the result of antenatal care improvements.
[For more information on the limitations of the CHW program, see the Challenges article in this narrative, in addition to the Exemplars narrative on CHWs in Bangladesh ().]
Neonatal Tetanus
In 1999, the Maternal and Neonatal Tetanus Elimination initiative was launched by UNICEF, WHO, and UNFPA to reduce cases of neonatal tetanus to less than one case per 1,000 live births in all districts within countries that had not achieved the standard for elimination, including Bangladesh. In the same year, the MOHFW began supplementary immunization activities in high-risk areas, with technical and financial support from WHO and UNICEF.
During the case study period, Bangladesh continued to implement facility-based tetanus toxoid vaccination sessions for pregnant women attending ANC sessions, as well as monthly outreach to ensure high coverage of tetanus toxoid vaccine for all women of reproductive age.
As a result of these and related efforts, Bangladesh achieved Maternal and Neonatal Tetanus Elimination status in 2008. According to IHME estimates, U5M attributable to tetanus in Bangladesh fell from 176 deaths per 100,000 children under five in 1990 to 4 in 2016, a 99 percent decline.
Neonatal Sepsis
Historically, management of neonatal infections in Bangladesh was limited to higher-level facilities such as district-level hospitals. A study conducted in India in 1999, however, demonstrated that such treatment was effective and feasible at lower levels of care, a finding that provided impetus for Bangladesh to consider the introduction of a similar initiative at the community level.
A 2007 study showed that community management of neonatal sepsis was feasible and acceptable, and within two years the government stated that neonatal sepsis should ideally be managed at facilities at the subdistrict level or higher, but community-level treatment was allowed if referral was not possible.
A study in 2014 found ongoing gaps in the effective management of neonatal sepsis, including lack of awareness by caregivers and CHWs of danger signs, and the persistent lack of skilled providers and poor quality of facility care.
Neonatal Resuscitation
In 2010, Bangladesh adopted the Helping Babies Breathe (HBB) initiative, an effort to reduce incidence of birth asphyxia, the leading cause of neonatal mortality in Bangladesh. This program, led by the American Academy of Pediatrics in collaboration with Saving Newborn Lives, WHO, the US Agency for International Development (USAID), the National Institute of Child Health and Development, and other global health organizations, sought to provide basic training on neonatal resuscitation in resource-limited settings. The HBB initiative in Bangladesh aimed to strengthen the capacity of both facility-level and community-level skilled birth attendants in neonatal resuscitation.
Bangladesh began implementing the HBB initiative with a small-scale testing phase in six districts – Habiganj, Noakhali, Lakshmipur, Jhalokathi, Bhola, and Pirozpur – with doctors, nurses, midwives, and CHWs participating in two days of training.
Assessment of the small-scale testing phase showed that participants’ scores on neonatal resuscitation knowledge tests increased from 85 percent before training to 99 percent afterward. The average number of correct bag-and-mask skills demonstrated by participants during simulated resuscitation increased from 1.7 (out of 7) before training to 6.7 during the post-training assessment.
These findings provided evidence both that providers’ neonatal resuscitation capabilities were unacceptably low and that the HBB training could effectively improve them.
In 2011, Bangladesh began a phased plan to expand HBB across the country, with financial support from USAID (through Save the Children) and UNICEF, as well as technical support from the USAID-funded Maternal and Child Health Integrated Program, Save the Children, Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh Pediatric Association, Bangladesh Neonatal Forum, and UNICEF.
The HBB initiative was scaled up to the national level in 2014. According to health facility survey data from that year, however, the readiness of facilities to provide neonatal resuscitation remained low, particularly at smaller, more localized facilities. Although 79.5 percent of district and subdistrict facilities had a neonatal bag and mask, only 34.7 percent of union-level facilities and 22 percent of community clinics had this basic equipment.
By 2017, HBB training was included as part of FB-IMCI. Informants reported that persistently low rates of skilled birth attendance (and facility-based delivery) continue to limit the country’s progress toward reducing birth asphyxia.
Clean Cord Care
In the 1990s and early 2000s, “dry cord care” – in which the stump of the umbilical cord following delivery is kept clean and dry, with little or no additional intervention – had become well established as the standard of care in Bangladesh.
Between 2007 and 2009, Bangladesh decided to assess the impact of two additional regimens of chlorhexidine cord care on cord infection and cord colonization, compared with dry cord care, in a randomized control trial designed to replicate a similar study conducted in Nepal.
The study had somewhat contradictory results. Although the single-dose group had a 20 percent lower NMR (23 deaths per 1,000 live births versus 28 for the dry cord care group), a longer treatment regimen did not reduce mortality. The multiple-dose regimen reduced severe cord infection, however, whereas the single dose did not.
As a result, in 2013 Bangladesh recommended a single application of 7.1 percent chlorhexidine in a newborn’s umbilical cord stump after cord cutting, followed by dry cord care in all births, regardless of the place of delivery. The comprehensive newborn care package developed that year included chlorhexidine use for cord care.
Despite these recommendations and policies, as of 2014 chlorhexidine for cord cleaning was available in only 32 percent of surveyed delivery facilities. In 2018, following the end of the study period, informants mentioned that chlorhexidine was to be rolled out in an additional 24 districts with support from UNICEF.
Decomposition
A decomposition analysis undertaken in collaboration with the Institute for Health Metrics and Evaluation (IHME) at the University of Washington identified the top interventions and risk factors that contributed to reduction in under-five mortality in Bangladesh. These interventions and risk factors can be seen in the bottom two bars of the visualization below.
- Some of the largest contributors to reduction in under-five mortality were health system interventions, which were responsible for 42 percent of the reduction in under-five deaths from 2000 to 2017.
- Within this, vaccines (especially Hib vaccine, PCV, and DTP3) were a significant factor, and were attributed to 13.9 percent of the overall reduction.
- Beyond health system interventions, reductions in child growth failure were found to be responsible for 13 percent of the reduction in under-five deaths over this time period, while decrease in incidence of low birth weight and short gestational age were found to be responsible for another 10 percent of the reduction.
