Key Points
The government shifted from an emphasis on disease-specific “vertical” strategies to a more holistic “horizontal” approach represented by the Integrated Management of Childhood Illness (IMCI). While this transition encountered some difficulties, IMCI was a centerpiece of Peru’s generally successful attempts to curb leading causes of mortality among children under the age of five (U5M).
Peru’s strong community health workforce and volunteers capably delivered the IMCI model into Peru’s remote towns and villages, in close cooperation with nongovernmental organizations (NGOs).
Vaccination campaigns were among Peru’s most successful and important interventions toward reducing U5M. The effectiveness and equity of these campaigns directly resulted from the government’s consistent policy of focusing on high-risk areas and areas most in need of early vaccine rollouts.
The research identified a series of evidence-based interventions that contributed to reductions in child mortality, including IMCI at the facility and community levels, malaria prevention and treatment, and expanded access to and quality of antenatal care, facility-based delivery, and postnatal care.
These and other evidence-based interventions are summarized in the sections that follow.
Integrated Management of Childhood Illness
- Peru used IMCI as part of a shift away from “vertical” disease-specific interventions and toward “horizontal” cross-cutting strategies and a focus on primary care.
- IMCI helped Peru improve diagnosis and care across several diseases and conditions.
- Despite some initial delays related to competing priorities between IMCI and existing vertical programs, integration of those vertical programs into IMCI has improved.
In 1995, the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) developed the IMCI strategy to guide the prevention and treatment of the most common causes of illness and death among children, including diarrhea, pneumonia, and malaria.
Facility-Based Integrated Management of Childhood Illness
Facility-based IMCI (FB-IMCI) focuses on strengthening the ability of health care providers to diagnose and treat conditions following simplified protocols, and on improving family and community health behaviors through education and practical assistance.
Peru was an early adopter of IMCI, deciding to implement the program in the same year that WHO and UNICEF had introduced it. The country suffered from high levels of childhood mortality and illness due to respiratory disease, diarrhea, and malnutrition, and experienced a high malaria caseload in certain regions.
The highly integrative nature of IMCI had a strong appeal for Peruvian health officials, who had encountered setbacks in trying to address each disease or condition separately – a “vertical” approach distinct from the more “horizontal” cross-cutting strategies of IMCI.
“Each vertical program became completely inefficient,” said one interviewee about the rationale for Peru’s adoption of IMCI. “If a child came in the hospital with a cough and diarrhea, a doctor would evaluate him for coughing, and then he would tell the family to go to the diarrhea unit. In the end the mother was completely confused, and sometimes she received different suggestions from doctors in different areas.”
Another interviewee affirmed that Peru “very enthusiastically accepted” IMCI, saying of the program, “It made all the sense in the world. Before then, sick children went to a health facility and there was a healthy-children consult office, a diarrhea practice, a respiratory infections practice, a micronutrient practice, and with this the child’s care was split into fragments, it didn't make any sense. So IMCI what it did was to look at the child integrally.”
Small-scale testing of IMCI began in six districts in October 1996. Equity considerations figured prominently in the selection of the pilot districts. The districts were chosen from the regions of Junin, Pasco, and Huanuco, which had higher rates of overall infant mortality, as well as higher rates of respiratory disease, diarrhea, and malnutrition among children under five.
Two other provinces – Ferreñafe and Caylloma – in the regions of Lambayeque and Arequipa were selected because they were among the poorest in their regions. Another province, Callao, was chosen because its location just outside of Lima allowed for close review and prompt follow-up by federal health authorities.
Going one step further from the province to the district level, in 1997, the Ministry of Health (MOH) selected additional districts from the regions of Apurimac, Ayacucho, Ica, Puno, Tacna, and Ucayali for early implementation of IMCI. According to key informant interviews, the MOH had an eye toward expanding the program to all 34 of the country’s health districts by 2001 – an ambitious timetable that indicated Peru’s impressive degree of commitment to the program (key informant, in-person interview, 2019).
That commitment received further affirmation in 1999, when the MOH conducted an assessment comparing health worker performance in the IMCI districts to performance in other parts of the country. This assessment found that quality of diagnosis and care was generally higher in the districts implementing IMCI.
By 2005, the MOH had integrated its vertical programs for malaria, respiratory infections, and diarrhea into IMCI, institutionalizing it as a core strategy of the country’s 2003 Comprehensive Childhood Health Care Model.
By the conclusion of the study period in 2015, the proportion of deaths attributable to the main diseases covered by IMCI had dropped. These included an 88 percent reduction in the rate of deaths due to nutritional deficiencies, 77 percent for diarrheal diseases, 67 percent for lower respiratory infections, and a 90 percent drop in the already-low rate of deaths related to malaria.
This is not to say that IMCI was a complete success. Particularly in the early years of the study period, the program encountered some serious challenges. An assessment of IMCI implementation as part of a 2003–2005 multicountry evaluation identified several potential constraints to effective scale-up of IMCI in Peru. Constraints included insufficient training and follow-up, lack of institutionalization of IMCI, high staff turnover, and a continued reticence to give IMCI higher priority than traditional vertical programs.
Some of these difficulties eased over time, but IMCI remained unable to conquer some of Peru’s most intractable health problems. For example, progress in care-seeking was inconsistent during the study period. Whereas the proportion of caregivers who reported seeking care at a health facility for children with fever increased from 35 percent in 1996 to 60 percent in 2014, the proportion seeking care for diarrhea was 32 percent in 1996 and 45 percent by 2009, but then dropped to 33 percent in 2014. For signs or symptoms of respiratory illness among children, care-seeking at health facilities improved from 49 percent in 1996 to 74 percent in 2012, but dropped to 60 percent by 2014. There was only limited improvement in the equity gap for care-seeking by wealth quintile between 2000 and 2016.
In addition, Demographic and Health Surveys (DHS) over the course of the study period showed that beyond care-seeking, the proportion of children receiving treatment for major conditions remained relatively unchanged. For example, the proportion of children under five with fever who received antibiotics remained low, from 22 percent in 2006 to only 23 percent in 2012. Nor did IMCI fully address the treatment challenges for diarrhea, another major cause of U5M specifically targeted by the program. The 2015 DHS reported that use of oral rehydration salts (ORS) stayed at modest levels over the study period, going from 36 percent in 2000 to 32 percent in 2015, with strikingly lower rates in regions such as Tacna (14 percent).
In 2014, the Ministry of Health provided an update to the original clinical guidelines published in 2006, standardizing the management of the diagnosis, treatment, and prevention of diarrhea in children. Also in 2014, Peru incorporated zinc supplementation in the standard treatment guidelines for diarrhea.
Community-Based Integrated Management of Childhood Illness
- Community-based (CB-IMCI) was successfully adopted countrywide, in part because of the country’s long-standing network of community health agents (called promotores in Peru).
- The role of promotores expanded substantially over the study period.
- The promotores initiative also benefited from strong support from NGOs and other partners.
Peru’s IMCI program was originally focused on facility-based interventions. Early on, however, officials recognized the need for community-based IMCI, or CB-IMCI.
“When we met, we said that it could not only be the clinical part – the most important part of child care is in the community-management component,” said one interviewee.
CB-IMCI in Peru was designed to incorporate family and community practices to promote infant and child health through community education, mobilization, home-based care, and referrals.
As an interviewee pointed out, Peru already had a crucial component of community-based care in place – a long-standing network of community health agents, known in Peru as promotores de salud (health promoters).
"We have a rich experience of [deploying health workers],” the interviewee said, observing that these workers often focused on individual, vertical programs. “The challenge was to change the logic toward a comprehensive vision, [where] the promotores are going to make a comprehensive family visit, and not only see the child, but see the child's surroundings.”
In this manner, the health workers would focus on the vertical programs, while the promotores would cover a broader set of health areas contributing to CB-IMCI.
CB-IMCI implementation began with training of promotores in 1997.
Trained promotores made monthly visits to households identified as “high risk”. This risk identification was conducted locally, using indicators such as mother’s education level, vaccination status of children under 5, children under 5 with a Control of Growth and Development (Control de Crecimiento y Desarrollo-CRED) card, access to safe drinking water, and adequate waste management.
Promotores provided families of children with referral slips and also received “counter-referral” feedback forms from facilities upon diagnosis and treatment of the referred child. Using these forms, promotores were able to assist mothers in care of their children and ensure compliance with prescribed treatment regimens.
The role of the promotores soon expanded to include promotion of hand-washing and hygiene, nutrition education, first aid services, and the monitoring of nutrition status for children, pregnant women, and postpartum women. In some regions, traditional birth attendants received training to serve as promotores.
The Good Start program also trained promotores on growth monitoring and psychosocial development of children. This program was a collaboration between the Peruvian government, USAID, and UNICEF, and monitored vitamin A deficiency and provided supplementation for children under five twice yearly in an effort to reduce chronic malnutrition. In addition, the Child Nutrition Program (Programa de Nutrición Infantil) worked with the promotores to improve health and nutrition through regular growth monitoring of children and educating mothers on appropriate nutrition practices. To aid these efforts, the Community Health Surveillance System (SIVICO) provided charts and maps of the entire community in relation to the status of health, nutrition, water, and sanitation.
The MOH worked with various organizations and NGOs to improve community access to primary health care services. NGOs such as CARE, Carita, Prisma and Plan International implemented CB-IMCI in different parts of the country under the leadership of the MOH, which provided the training for promotores and supervision through staff from health facilities.
Partner organizations including the Pan American Health Organization (PAHO), UNICEF, and the Peruvian Red Cross also participated in the training of promotores from the beginning of CB-IMCI in 1997. Instructional programs expanded rapidly, surpassing the training levels achieved by FB-IMCI. By 2001, approximately 2,500 promotores had been trained in CB-IMCI. At the time, approximately one IMCI-trained promotor was available per 500 children nationally.
Peru’s strategy of leveraging partner support for CB-IMCI contributed to its success. As one interviewee explained, “Compared to FB-IMCI, the community management (CB-IMCI) is the one that at the time was much stronger, because many partner organizations began to orient their work around this and promoted the community component.”
Malaria
- Peru’s anti-malaria efforts included an extensive mosquito bed net distribution campaign that encountered challenges in persuading households to switch from traditional non-treated nets to long-lasting insecticide-treated nets.
- The country was an early adopter of rapid diagnostic testing, although it faced problems with promotores administration of the test and supply chain failures that hampered delivery at the community level.
- The country also swiftly adopted artemisinin-based combination therapy, with successful distribution in rural areas most heavily hit by malaria.
While real but limited gains were realized against two of the primary targeted diseases of IMCI (pneumonia and diarrhea), somewhat more impressive gains were realized in reducing malaria.
The mosquito-borne disease has long been a particular challenge for Peru, and it remains so. As of 2016, the country still accounted for 14 percent of all reported cases of malaria in the Western Hemisphere.
In Peru the primary cause of malaria has historically been the protozoal parasite P. vivax, with P. falciparum causing less than 1 percent of all cases in the country. Annual reported cases of malaria in Peru steadily declined from 2005 to 2010, by 63 percent for P. vivax and 95 percent for P. falciparum. Malaria incidence climbed after 2011, but malaria mortality among children under five did not experience an associated increase during this period. This seems to have been primarily a result of successful IMCI-driven efforts to expand diagnosis and treatment.
During the study period, the majority of malaria cases occurred in Loreto, Peru’s northernmost region and by far its largest. With its thick Amazonian jungles and a population of only about 1 million people, Loreto covers nearly a third of Peruvian territory and represents the country’s primary malaria-endemic area.
Bed Net Distribution Campaigns
Peru’s largest public distribution campaign of long-lasting insecticide-treated nets (LLINs) and conventional insecticide-treated nets (ITNs) began in 2006 with support from the Andean Health Plan for the Borders (PAMAFRO or Plan Andino de Salud en Fronteras), which was supported by the Global Fund to Fight AIDS, Tuberculosis and Malaria. The Ministry of Health (MOH) and PAMAFRO conducted the campaign in high-risk malaria areas of the Amazon jungle (Amazonas, Loreto, and Cajamarca).
Distribution areas were selected based on several factors, including disease burden and lack of access to health services. The campaign made a focused effort to reach certain population categories within these areas, including children between three and ten years of age, parents with children under age three, and pregnant women.
Within a year, 85 percent of households in priority distribution areas owned an insecticide-treated net. However, most of these nets had been acquired by the households themselves, not from the distribution campaign, and they were often in poor condition.
A 2007–2008 study of ten communities indicated a popular preference for untreated traditional nets. It found that 63 percent of households did not use any of the LLINs that had been previously distributed. The majority of respondents in the study reported that mosquitoes had entered their LLIN since the beginning of its use or after the net’s first wash (93 percent) and that mosquitoes did not die after entering the net (90 percent). Overall, 94 percent believed that traditional nets offered better protection than LLINs. In addition, traditional nets provided an added degree of privacy, a function that was limited for the more transparent LLINs.
Distribution of LLINs continued until 2010 – by then, the campaign had distributed over 242,000 LLINs. A study conducted in 60 communities found that shortly after distribution, 99 percent of children under age five and 96 percent of pregnant women had slept under an LLIN the night before data collection. However, lower use was reported approximately one year after distribution, when only 77 percent of children under five and 66 percent of pregnant women were found to have slept under an LLIN the night before.
Rapid Diagnostic Testing
Peru was an early adopter of rapid diagnostic tests (RDTs) for malaria. Its first study of the technology, an efficacy trial conducted in 1999, equipped 20 promotores with RDTs. The promotores performed rapid tests and also prepared blood smear slides for microscopy for approximately 400 febrile patients. The study found that the RDTs administered by promotores achieved high sensitivity and specificity.
A later study conducted from September 2001 to May 2003, which also equipped promotores with RDTs, found that the median time between a patient’s initial visit to a promotor and receipt of diagnosis decreased from 68 hours before the use of RDTs to only 20 minutes. The proportion of confirmed cases treated within two days of symptom onset increased from 16 percent to 55 percent. In addition, patients were more likely to receive the appropriate treatment for the parasite species they were infected with (84 percent, compared with 27 percent before use of RDTs).
Implementation of RDTs at the community level could also result in financial savings, due to both the elimination of overtreatment and a reduction in the number of complicated cases due to more timely and species-specific diagnosis. Specifically, RDT implementation was estimated to save the MOH US$191 per case of P. falciparum and US$31 for each case of P. vivax malaria.
During a workshop held in July 2003, local health workers involved in the trial discussed facilitating factors and barriers to effective RDT use. They identified a range of problems including stock-outs, community distrust generated by false-negative results, the inability of the test to detect low levels of parasitemia, and the limited skills of promotores at taking finger-stick samples and reading RDTs correctly. Despite these challenges, health workers recommended the expanded use of RDTs.
Based on these and other findings, the MOH decided to implement community-based RDTs in areas with limited access to microscopy. However, it did not establish guidelines defining areas of implementation. According to an in-country expert (Dr. Patricia Garcia, written communication, February 2020), despite Peru’s early adoption, large-scale, sustained use of RDTs was a challenge, especially after Global Fund financing declined The MOH and PAMAFRO have not procured or distributed any RDTs since a large procurement of tests in 2007. Though UNICEF has since donated a small amount of RDTs in the Amazonas Region, large-scale national or even regional distribution has not occurred.
This lack of RDT availability at the community level was exacerbated by lack of acceptance among facility-based health workers. Some health workers did not trust promotores to provide these services and were sometimes reluctant to supply them with RDTs, as noted by an in-country expert (Dr. Patricia Garcia, written communication, February 2020).
Artemisinin-Based Combination Therapies
In 2001, Peru become the first country in Latin America to implement artemisinin-based combination therapies (ACTs) for treatment of malaria, five years before WHO issued recommendations for the use of ACTs in treatment of uncomplicated P. falciparum malaria issued in 2006. Peru was also the first country to simultaneously use two ACT schemes as first-line treatment in different areas based on subnational resistance data.
Trainings first targeted health workers at the regional level, then were cascaded down to local levels in the following months. Regional health authorities trained physicians, nurses, and laboratory specialists at health centers and health posts. ACTs were subsequently provided as treatment for P. falciparum malaria at health facilities as well as at the community level by promotores.
PAMAFRO implemented a strategy to acquire antimalarial drugs for participating countries – Peru, Venezuela, Bolivia, and Colombia. During PAMAFRO’s first two years of implementation, unit prices for these drugs, including ACTs, steadily dropped. For example, the price of one unit of artesunate dropped by about 90 percent due to the PAMAFRO strategy. To minimize corruption and ensure availability of ACTs at the community level, PAMAFRO hired suppliers to distribute ACTs directly to all health centers in rural jungle areas.
Vaccines
- One of the primary drivers of Peru’s reductions in U5M has been the success of the country’s vaccination campaigns. One clear example of that success is that no indigenous case of measles has developed since the start of the study period in 2000, even as the disease remained present in neighboring countries.
- Peru has also curbed other vaccine-preventable illnesses, and this achievement has been central to the country’s overall reductions in U5M.
- A notable feature of Peru’s vaccination campaigns is the practice of piloting or introducing vaccines first in poorer regions, especially for rotavirus, pneumococcal vaccine, and Hib – a tangible sign of the country’s emphasis on reducing equity gaps in health care.
Rotavirus Vaccine
A 2001 study estimated more than 63 percent of Peru’s children would experience an episode of rotavirus diarrhea by the age of five, and that 1 in every 375 would die of the virus.
A 2008–2009 study reported that Peruvian hospitals lacked rapid diagnostic tests for rotavirus, elevating the risk of unnecessary antibiotic prescriptions and additional disease outbreaks. Before introduction of the rotavirus vaccine in 2009, approximately 384,000 cases of rotavirus occurred each year, resulting in 64,000 clinic visits, 30,000 hospitalizations, and 1,600 deaths. The medical care for these rotavirus cases cost approximately US$2.6 million per year, excluding indirect or societal costs. Among those costs was an increased risk of malnutrition, which was found to correlate closely with incidence of rotaviral diarrhea.
“Up until 2009 the country did not vaccinate against rotavirus,” recalled one interviewee. “However, the evidence told us that to reduce malnutrition, we needed to reduce acute respiratory infections and diarrhea in children, which has a much more direct effect on malnutrition than the issue of delivering food.”
According to a key MOH informant (interview, March 2019), after recognition of the need to address rotavirus, Peru invested US$400 million in the purchase of vaccines for it – a major example of the country’s willingness to make serious investments in health initiatives. Upon its full integration into the national immunization schedule in 2009, the rotavirus vaccine was intended to be administered at two and four months of age, with an emphasis on rolling it out first in the poorest regions of the country.
By the end of 2009, national coverage for receiving both doses of the vaccine had already reached 41 percent, and resulted in 77 percent savings in government spending on the disease. Coverage quickly climbed, reaching 91 percent in 2012. Peru reached 87 percent national coverage of rotavirus vaccination in 2015.
Pneumococcal Conjugate Vaccine
In 2000, 6,174 Peruvian children under the age of five died of pneumonia – approximately 20 percent of all under-five deaths. Of these pneumonia-related deaths, at least 30 percent – and perhaps as many as 50 percent – were attributed to pneumococcus. An estimated five to eight children under the age of five died in Peru each day in 2000 due to pneumococcal disease.
To address this crisis, Peru licensed the PCV7 variant of the pneumococcal conjugate vaccine in 2000. In addition to potentially saving thousands of children’s lives, an effective pneumococcus vaccine could also save money; the MOH estimated the cost of treating a single case of mild infant pneumonia was somewhere between US$104 and US$156. However, two doses of PCV cost only US$69.
Despite its early licensing of the vaccine – and the clear humanitarian and financial incentives for rolling it out – the National Vaccination Scheme did not introduce it until 2008; according to an in-country expert (Dr. Patricia Garcia, written communication, February 2020), this was due in part to insufficient funding and conflicting government priorities. The following year, the MOH finally added PCV to the national immunization schedule, with administration at three months, five months, and 12 months of age.
As with rotavirus, Peru made a point of introducing PCV first in the poorest areas of the country. One interviewee explained that while the MOH intended from the start to implement PCV nationally, introducing the vaccine in high-priority areas allowed it to build evidence and gain support for national rollout. By 2010, PCV was released countrywide.
In 2011, PCV7 was taken off the global market and replaced with the PCV10 and PCV13 presentations, which covered additional serotypes. The Peruvian National Institute of Health conducted two studies to inform the MOH’s decision on which presentation to introduce.
The first study, conducted in 2011, found that both PCV10 and PCV13 would be more cost-effective than PCV7 in preventing pneumonia in children under five. PCV10 would cover 71 percent of pneumococcal isolates, and PCV13 would cover 82 percent. In addition, the evaluation estimated that while PCV13 would have a higher initial price tag than PCV10, it would be more cost-effective in the long run because of its greater potential for reducing hospitalizations. However, Peru ultimately opted for PCV10 over PCV13, introducing the vaccine in late 2011.
Peru soon achieved high coverage of PCV. Coverage of three doses of PCV reached 89 percent by 2012, just one year after the country’s transition to PCV10. It was scaled up to the national level in 2013. The MOH waged a catch-up campaign, with two doses of the vaccine for unvaccinated children between 12 and 24 months of age, and one dose for children 2 to 5 years of age.
Despite shifts in the implementation of PCV in Peru during the study period, the country maintained high levels of coverage. By 2015, PCV coverage reached 90 percent. One year later, the MOH incorporated PCV13 into the national immunization schedule to protect against additional serotypes.
Haemophilus Influenzae Type B
In 1998, the MOH incorporated the Haemophilus influenzae type b (Hib) vaccine into the National Scheme for Vaccination as a routine intervention for children under age five in the poorest areas of the country. At the beginning of the study period in 2000, Hib-related meningitis caused only 0.39 percent of under-five deaths in Peru, but this greatly underestimated Hib’s overall risk. Lower respiratory infections accounted for an estimated 20 percent of all U5M in the country during the same year, and Hib was among the causes of those infections.
During its initial use in Peru, the Hib vaccine was introduced only in selected geographical areas of the country due to limited resources and the country’s interest in prioritizing low-income areas for vaccine rollouts. The Hib vaccine became part of routine vaccination for children living in priority areas of extreme poverty, with doses given at two, three, and four months of age.,, In 2005, the vaccine was introduced nationally.
In alignment with new international standards, Peru first introduced the DTP3 pentavalent vaccine containing Hib in selected areas of the country in 2005. By 2007, the pentavalent vaccine was scaled up to the entire country and provided at two, four, and six months of age.
Peru quickly achieved widespread national coverage of the pentavalent vaccine, and coverage remained high throughout the study period, with some geographic variation. The 2014 DHS showed that DPT3 pentavalent coverage was nearly identical in urban (77.8 percent) and rural (77.9 percent) areas, but it differed by region, ranging from 61.2 percent in Madre de Dios to 86.5 percent in Tumbes.
Overall, across all vaccines, statistical analysis found that 13.3 percent of the reduction in under-five deaths was attributable to vaccine interventions, including PCV (6.0 percent), Hib vaccine (5.2 percent), and rotavirus vaccine (2.1 percent).
HIV
- To prevent mother-to-child transmission of HIV, Peru successfully increased HIV testing during antenatal care, antiretroviral therapy for mothers, and distribution of formula to HIV-positive mothers.
- On-site testing became part of routine antenatal care, which helped increase access to testing.
- Nonetheless, gaps in screening and diagnosis remain, and HIV deaths among children under five were on the rise at the end of the study period, but subsequently have been declining.
In 2002, the MOH estimated that 0.3 percent of pregnant women in Peru were HIV positive. In September of that year, Peru submitted a proposal to the Global Fund seeking funds for activities to control HIV/AIDS and tuberculosis. As part of this proposal, the MOH established a goal of maintaining or decreasing the prevalence of HIV within five years.
The MOH sought to cut the rate of mother-to-child HIV transmission – sometimes referred to as “vertical” transmission or MTCT (for “mother-to-child transmission”) – to 8 percent or less. Toward this end, it aimed to increase the percentage of pregnant women receiving HIV tests at antenatal care (ANC) visits from 30 percent to 85 percent.
Pregnant women diagnosed with HIV during pregnancy would receive antiretroviral therapy (ART) and would be provided with artificial milk for the child’s first six months of life. As a result of this proposal, the Global Fund granted Peru US$6.5 million in support for HIV and TB activities in 2003.
Beginning in 2004, the government began to focus increasingly on implementation of activities for the prevention of mother-to-child transmission (PMTCT), with support from the Global Fund. That year, it passed a law making HIV testing obligatory for pregnant women countrywide.
The MOH soon began to offer rapid on-site testing as part of ANC. Before the introduction of these tests, access to screening was limited and delays in confirming diagnoses and initiating treatment were common.
An interviewee explained that introduction of rapid tests was a vital step in scaling up PMTCT efforts in Peru, stating that it “marked a milestone in terms of being able to improve access to the screening of pregnant women, which was the first step in being able to intervene.”
In 2005, with support from the Global Fund, the MOH began to provide free ART, including prophylaxis for infected pregnant women and treatment for children. ART in Peru was initially offered at only well-equipped hospitals in large cities, restricting access to these services. This situation improved due to decentralization of treatment to more regions in subsequent years, although it is still offered primarily in hospitals or larger health centers.
As part of its national family planning initiatives, the MOH adapted its HIV screening approach in 2008 to target women of childbearing age before pregnancy. This approach facilitated testing of over 2 million women in Peru, increasing the coverage of HIV testing in women from only 12 percent in 2000 to 50 percent in 2012.
Among pregnant women, the gains were just as impressive, with testing increasing from 23 percent in 2000 to 80 percent by 2011. Coverage varied at the regional level throughout the study period, however, due to recurring shortages in testing supplies and problems in the supply chain.
Improvements in testing coverage were accompanied by improvements in ART coverage for pregnant women. Overall coverage of ART treatment in pregnant women increased from 20 percent in 2000 to 59 percent in 2012. However, alternative estimates using health service data show that 92 percent of HIV-positive pregnant women received ART for prophylaxis or treatment in MOH-run facilities by 2010. Accordingly, estimated maternal-to-child transmission rates in Peru dropped from 15 percent in 2005 to 9.1 percent in 2009.
A statistical analysis from the Institute of Health Metrics and Evaluation (IHME) corroborates these improvements in coverage, attributing 5.9 percent of the overall reduction in U5M to the combination of prevention of mother-to-child transmission (3.2 percent) and ART for children (2.7 percent) .
Despite progress in coverage of screening and ART in pregnant women and subsequent reduction of mother-to-child transmission, both testing of children born to HIV-positive mothers and treatment of HIV-positive children lagged far behind. In 2011, only 10 percent of exposed infants were tested within two months of birth.
Treatment rates for diagnosed children also remained low, at about 18 percent in 2013. The total number of infected children in the country reported by diagnostic and laboratory services dropped from 5,600 in 2000 to 3,200 in 2012. However, this decline may be at least partially related to reduced screening coverage during this period rather than solely a drop in pediatric cases.
While HIV accounts for less than 1 percent of Peru’s U5M, the estimated number of HIV-related deaths among children under five increased toward the end of the study period, albeit with subsequent decline in the years following.
Key informants noted that this increase is likely due to improved data quality and increased reporting, rather than actual increases in child deaths due to HIV. The Joint United Nations Programme on HIV/AIDS (UNAIDS) estimates the number of AIDS-related deaths among Peruvian children up to 14 years of age has decreased continually during the study period – from approximately 500 in 2000 to about 100 by 2015.
Neonatal Interventions
- Peru’s 51 percent reduction in neonatal mortality during the study period reflects the successful deployment of a range of evidence-based interventions, from the antenatal to the postnatal phase.
- One striking element of Peru’s efforts to reduce neonatal mortality is the extent to which the government and development agencies worked to account for widely varying cultural norms pertinent to neonatal health – especially with regard to facility-based delivery and birthing practices.
- An important gap in neonatal health at the start of the period was in emergency obstetric and newborn care. Peru embarked on a collaboration with nongovernmental organizations and university partners to successfully improve access to care, starting with one region, and with aspirations to build on this progress countrywide.
Antenatal Care
The 2000 DHS reported that 69 percent of Peruvian women who had a live birth in the previous five years attended at least four antenatal care visits (the number recommended by the WHO at the time), and 84 percent attended at least one. While these figures are relatively high, Peru succeeded in improving upon them over the study period to almost universal coverage.
One way it succeeded was setting a higher bar for itself – by recommending that pregnant women attend at least six ANC visits, and that those with high-risk pregnancies attend at least eight visits.
The MOH created a national intervention plan for maternal health care, which guided efforts to implement the six-visit protocol in Peru from 2009 to 2015. In addition to encouraging beneficiaries of the Juntos conditional cash transfer program (more detail here) to attend ANC visits as a condition of receiving a cash transfer, the government also included ANC services in the national health insurance system (Seguro Integral de Salud or SIS). Beginning in 2001, this system covered six or more ANC visits (including home visits), along with complete laboratory screenings, two ultrasounds, and preventive treatment with iron sulfate.
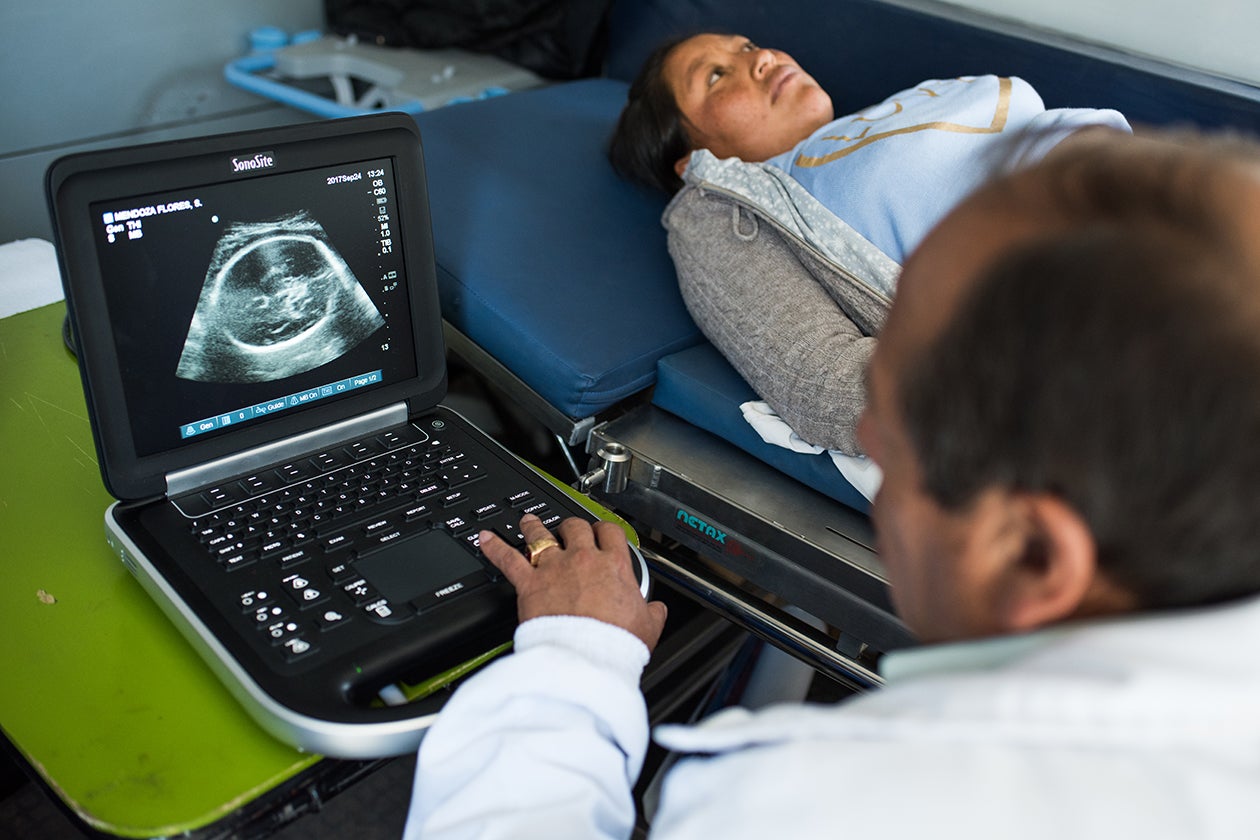
Another important ANC program was Peru’s Salud Materno Neonatal, designed to reduce maternal and neonatal mortality by strengthening the health system’s ability to provide a basket of critical services for women and newborns and cultivating health-seeking behavior in rural women. This program increased the number of women receiving such vital services as folic acid supplements, fetal growth monitoring, and at least four ANC visits.
An independent 2017 evaluation drawing upon DHS data from 2000 to 2014 found that eligible women participating in the Juntos program during their most recent pregnancy had a 2.9 percent higher rate of attending at least one ANC visit – and 3.8 percentage points more likely to attend at least four visits – than women who were not in the program. In addition, women in Juntos were 7.7 percentage points more likely to receive ANC within their first trimester.
The 2012 DHS reported improvements in the use of antenatal care services in Peru. Overall, 94 percent of pregnant women attended at least four visits, and the proportion who did not attend any ANC visits at all was only 2 percent. The 2012 DHS also reported that 74 percent attended their first ANC visit within the first trimester of pregnancy; in 2000, only 57 percent had done so. Furthermore, equity plots demonstrate that the gap between wealth quintiles in ANC attendance sharply decreased over the study period.
While Peruvian women are attending a relatively high number of ANC sessions, at least one interviewee expressed concern about the quality of care they are receiving.
“Prenatal checks, for example, are not good quality,” the interviewee said. “There has been an effort to improve this, but it has not yet been achieved. Just because you have eight prenatal checkups doesn’t necessarily mean you’ve solved the maternal health problem.”
Improving Childbirth Delivery Services, Access, and Uptake
Skilled Birth Attendance
In 2000, the MOH launched its Maternal and Perinatal Program, which aimed to promote and increase coverage of facility-based delivery or home delivery attended by trained health personnel. A study that year found that only 62 percent of live births in Peru over the preceding three years had been assisted by a skilled provider (defined as a doctor, nurse, auxiliary nurse, or midwife). There were large geographic variations, with 87 percent of urban deliveries but only 29 percent of rural births assisted by a skilled provider.
The MOH recognized the need for traditional midwives to collaborate with trained health personnel to increase skilled birth attendance countrywide. The MOH released a set of guidelines in 2000 that aimed to prepare traditional midwives to more efficiently recognize complications related to pregnancy, delivery, the postpartum period, and in newborns.
These and other policies resulted in an increase in skilled birth attendance. A comparative study found that while 58 percent of deliveries occurred at home and 6 percent at health centers as of 1999, deliveries attended by skilled providers increased to 95 percent by 2007.
In addition, the study found improvements in the working relationship between traditional birth attendants, other community health agents, and health center professionals, with the traditional birth attendants increasingly seeking expert help and even referring women and newborns to health centers.
The 2014 DHS also showed improvements in the coverage of skilled birth attendance; 91 percent of women who gave birth in the previous three years had received assistance from a skilled provider during delivery – an impressive increase from only 62 percent in 2000.
A study published in 2013, however, demonstrated an ongoing preference for traditional birth attendants among some populations. It found that just half of Quechua mothers preferred to be supported by formal health care providers during delivery, while the other half chose to be assisted by traditional midwives.
Facility-Based Delivery and Cultural Adaptations
At the start of the study period in 2000, only 58 percent of women in Peru giving birth delivered at a facility, and regional variation was significant. Although facility-based delivery was as high as 90 percent in Lima, it was below 30 percent in several regions, including Amazonas (27 percent), Cajamarca (22 percent), Huancavelica (20 percent), Huanuco (28 percent), and Puno (20 percent). Only 13 percent of Quechua speakers delivered in facilities, and 12 percent of speakers of Aymara.
To address the highly variable rates of facility-based delivery in Peru, the MOH implemented new policies and programs to improve access to birth facilities and encourage women to use them. In 1996, the MOH launched Proyecto 2000, funded by the United States Agency for International Development (USAID), which sought to make birth-facility services more accessible, convenient, attuned to cultural norms, and respectful of patient confidentiality.
To inform implementation of Proyecto 2000, members of the implementation team and regional MOH educators conducted a qualitative baseline study on mothers’ perceptions and preferences related to childbirth. Drawing upon that study, Proyecto 2000 launched a Safe Motherhood multimedia campaign to increase demand for facility-based delivery services.
The program trained nearly 3,700 promotoras (female community-health agents) to support these activities. In addition, health facility staff engaged with community health committees to promote facility-based delivery.
In 1998, Proyecto 2000 conducted an evaluation, which found that mothers receiving care at Proyecto 2000 facilities were more knowledgeable about pregnancy, more satisfied with their care experience, and also more likely to deliver in that same facility than mothers who received care elsewhere. A study conducted in 2006, however, found that Proyecto 2000 likely improved quality of care offered at health facilities, but did not directly influence the probability of facility-based delivery.
Beginning in 2004, the MOH implemented new delivery standards in health facilities to provide more culturally sensitive services. These facilities then began to accommodate the traditional vertical birth position, during which the mother is standing or squatting – a widespread practice in Peru’s rural areas – rather than lying down.
Following this change, facilities set up birthing rooms where women could deliver in a standing or squatting position, with their husbands and other family members present. In 2005, the MOH adopted a technical standard allowing for vertical delivery.
In addition to addressing financial barriers and improving quality, the MOH focused on alleviating challenging geographic impediments to facility-based delivery, particularly in rural areas. As part of this strategy, in 2004 the government (along with UNICEF and USAID) created maternal waiting homes, houses in which women could stay until the time came to deliver at nearby facilities. By 2006 there were 337 maternal waiting houses in the high Andean areas alone. Overall, by 2008, Peru had established 390 maternal waiting homes.
One interviewee noted that not all health care providers adopted this change, particularly in large cities. “This attitude of helping women give birth in the way she wants was not something all professionals did.”
As a result of these implementation strategies, Peru achieved impressive improvements in facility-based delivery rates during the study period. The 2012 DHS reported that 87 percent of deliveries over the preceding three years had taken place at a health facility.
Despite this success, some equity differences persisted. While 96 percent of women in urban communities delivered at a facility, only 68 percent of women in rural areas did so.
Regional differences were also still evident; the facility-based delivery rate was highest in Ica at 99 percent, and lowest in Amazonas at only 59 percent. Although by 2014, facility-based delivery rates in Amazonas increased to 70 percent.
Equity plots show that rates of facility-based delivery increased across all wealth quintiles in Peru during the study period. The equity gap between coverage of the different wealth quintiles also narrowed over the study period. However, a notable disparity between the poorest and all other wealth quintiles remained in place.
Emergency Obstetric and Newborn Care
Emergency obstetric and newborn care (EmONC) was first introduced by WHO, the United Nations Children’s Fund (UNICEF), and the United Nations Population Fund (UNFPA) in 1997 as a strategy to reduce maternal and newborn mortality, especially in low-resource settings. Basic emergency obstetric and newborn care (BEmONC) consists of seven key services known as signal functions:
- Administration of parenteral antibiotics
- Administration of parenteral anticonvulsants
- Administration of parenteral uterotonics
- Removal of retained products
- Assisted vaginal delivery
- Manual removal of the placenta
- Newborn resuscitation
Comprehensive emergency obstetric and newborn care (CEmONC) includes the seven basic signal functions in addition to surgical capability (primarily for provision of cesarean sections) and blood transfusion.
Availability and use of high-quality EmONC services has been estimated to prevent between 40 percent and 62 percent of neonatal deaths. However, use of these services in Peru was less than ideal – a 2000 survey conducted in the Ayacucho Department found that only about a quarter of pregnant women with complications gave birth at CEmONC facilities that were considered “adequate.”
At the national level, the 2000 DHS found that approximately 83 percent of women faced at least one barrier to accessing EmONC-compliant health services. Common barriers to access included cost of treatment, lack of female caregivers, cultural and language barriers, lack of support from health care providers, inefficient management of obstetric emergencies, and emotional mistreatment by health care providers.
In 2000, CARE Peru began implementing the Foundations to Enhance Management of Maternal Emergencies (FEMME) project with support from the Bill & Melinda Gates Foundation and the Averting Maternal Death and Disability program at Columbia University. The project aimed to increase access, availability, and use of EmONC services for about 48,000 pregnant women in the northern region of Ayacucho.
Examples of FEMME’s success include working with hospital staff to provide services attuned to local cultural practices, such as the adoption of vertical delivery birthing chairs and allowing family members to be present during delivery. In addition, FEMME worked with the MOH to ensure that emergency drugs, basic equipment, and supplies essential for EmONC were available at facilities. To improve the technical capacity of providers, FEMME staff provided advanced clinical training in emergency obstetric interventions and use of standardized protocols, followed by post-training supervision visits. Overall, according to an in-country expert (Dr. Patricia Garcia, written communication, February 2020), due to the project’s success with improving access to EmONC services in northern Ayacucho, the program was taken to the MOH and efforts to scale up the program nationally were initiated in 2009.
A 2010 assessment released by the Instituto Nacional De Estadistica e Informatica found still limited EmONC capacity at facilities across Peru. This assessment determined the level of capacity as measured by the availability of basic obstetric and neonatal functions in over 250 facilities in 13 departments. To reduce maternal morbidity and mortality, facilities were expected to obtain capacity for at least 80 percent of these functions.
The assessment instead found that only 0.4 percent of facilities had achieved this threshold and that most facilities (59 percent) had a capacity of less than 50 percent. An assessment conducted in 2015 found that by the same methodology and definition of resolutive capacity, there was no improvement in capacity of facilities.
Postnatal Care
Neonatal Resuscitation
Peru’s Neonatal Resuscitation Initiative was implemented by the Social Security System (EsSalud) beginning in 1999. EsSalud offers health services to 20 to 30 percent of the population, mainly those with formal jobs. The initiative trained 1,272 health care providers from 54 hospitals on neonatal resuscitation techniques using the American Academy of Pediatrics’ Neonatal Resuscitation Program (NRP).
Participating EsSalud hospitals implemented a protocol to reduce mortality from birth asphyxia. This protocol required all deliveries to be attended by NRP-trained personnel and that all complicated deliveries be attended by NRP-trained neonatologists.
Efficacy of the program was assessed based on data from one of the 54 intervention hospitals – Edgardo Rebagliati Martins Hospital in Lima. It was the largest EsSalud hospital and had both accessible and reliable data.
In 1998, before initiation of the program, the hospital had a neonatal asphyxia rate of 13.8 per 10,000 births. During the program’s implementation period from 1999 to 2004, the hospital’s neonatal asphyxia rate decreased sharply, to 3.39 per 10,000 births. This decline in birth asphyxia was sustained even after conclusion of the study, decreasing to 2.33 per 10,000 births in 2010.
Decrease in neonatal asphyxia rate at EnSalud hospital, per 10,000 births

Elsensohn A N, Ricks D J, et al. The success of Peru’s Neonatal Resuscitation Initiative. 2013. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.829.5871&rep=rep1&type=pdf. Accessed March 1, 2019
Beginning in 2004, Peru recognized the need to better train health care workers to properly care for neonates with asphyxiation, according to a key in-country informant (interview, March 2019). This informant also noted that doctors, obstetricians, nurses, and technical staff from the MOH who were already involved in responding to neonatal emergencies were trained on diagnosis, stabilization, and referral – steps that have been credited with helping reduce maternal and neonatal morbidity and mortality.
Neonatal Intensive Care Units
In 2005, the MOH established its Policy for Intensive and Intermediate Care Services, which established administrative procedures to improve the quality of care received in intensive care facilities. The following year, the MOH established a directive for evaluating neonatal care in health establishments, and defined the personnel, instruments, and equipment necessary for intensive obstetric and neonatal care.
The Strategic Maternal and Neonatal Health Program was one of the first results-based budget initiatives put forward by Peru’s Ministry of Economy and Finances. The program was developed in 2007 and scaled up in 2009 and included access to neonatal intensive care as a key indicator. In this program, the MOH, the Comprehensive Health Insurance program, and the regional governments all appropriated financial resources to improve access to neonatal services in neonatal intensive care units (NICUs).
The Ministry of Economy and Finances estimated that in 2009, 58.8 percent of neonates with health complications were being treated in NICUs in Peru, with the lowest coverage in Ayacucho (44.6 percent) and the highest in Cusco (66.7 percent).
In 2009 and 2010, in preparation for an H1N1 influenza outbreak, Peru began implementing NICUs across the country, including incubators and neonatal resuscitators. Because of this global epidemic, resources were allocated to address a potential crisis, and neonates in Peru benefited in the long term once the threat dissipated, since the supplies were kept in place for neonatal care (as noted in an interview with a key in-country informant, March 2019).
When asked what measures contributed to the decrease in neonatal mortality between 2000 and 2015, one key informant mentioned the procurement of NICUs as a primary contributor, saying, “I think the NICU has been better prepared, both in terms of the equipment they have and the clinical competency of the hospital staff who care for the children.”
However, NICU access remains “largely an ongoing effort” at the national level. When writing about the epidemiology of neonatal mortality in Peru from 2011 to 2012, key leaders at the MOH, UNICEF, and a regional hospital in Cusco attributed high mortality rates in the first seven days of life in part to the lack of NICU availability across the country. These leaders urged the establishment of NICUs and perinatal networks that take into consideration the level of care (basic, intermediate, or intensive) that the neonate requires.
Postnatal Visits
The 2000 DHS found that 72 percent of women who gave birth in a facility received postnatal care from a medical professional; however, among women who gave birth outside a health facility, only 33 percent received postnatal care.
In 2013, the MOH published a policy promoting integrated health care for neonatal health at the national level. This policy detailed clinical guidelines that recommended follow-up on the mother and infant within 48 hours of the birth, and then once per week, either in a health facility or at home.
The guidelines highlighted the importance of training professional health care staff to promote key practices with the newborn such as immediate breastfeeding, hand-washing, good hygiene practices, immunizations, and identifying danger signs in the mother and the infant.
The 2015 DHS reported improvements in postnatal care – 97 percent of new mothers had received a postnatal checkup, and 76 percent of those were within four hours of delivery.
Postnatal care rates improved in both urban and rural areas, though women living in urban areas (77 percent) were still slightly more likely than those in rural areas (72 percent) to receive postnatal care within four hours. Even more women countrywide (96 percent) received postnatal care within the first two days after delivery, though some regions still struggled to achieve high rates of coverage. In the Loreto and Amazonas regions, roughly one woman in five received no postnatal care at all .
Overall, statistical analysis attributed that 18.7 percent of the reduction in under-five deaths was due to maternal and neonatal care interventions – 2.7 percent of the reduction was due to the increased use of skilled birth attendants at delivery, and the other 16 percent was attributed to a decrease in incidence of low birth weight and short gestational age, which were likely to have been at least partly the result of antenatal care improvements .
Decomposition
A decomposition analysis undertaken in collaboration with the Institute for Health Metrics and Evaluation (IHME) at the University of Washington identified the top interventions and risk factors that contributed to reductions in under-five deaths in Peru. These interventions and risk factors can be seen in the bottom two bars of the visualization below.
Below are some of the key takeaways from this analysis:
- Some of the largest contributors to reduction in under-five mortality were health system interventions, which were responsible for 53 percent of the reduction in under-five deaths from 2000 to 2017
- Within this, vaccines (especially PCV, Hib, and rotavirus) were a significant factor, and were attributed to 13.3 percent of the reduction
- Beyond health system interventions, population age structure, reductions in child growth failure, and reductions in low birth weight were also found to have significant contributions to reduction in under-five deaths, with attributions of 22 percent, 16 percent, and 16 percent, respectively.
