Key Points
The government prioritized a shift from disease-specific "vertical" strategies towards a more comprehensive "horizontal" approach, as evidenced by the development of integrated community case management (iCCM) for diarrhea and pneumonia.
Rwanda deployed a variety of major programs to address malaria and malnutrition, prevent mother-to-child transmission of HIV, vaccinate against pneumonia and rotavirus, and improve neonatal outcomes.
Rwanda's community health workers played a key role in expanding coverage of these interventions.
Malaria treatment and prevention
Home-based management of fever (HBMF) and integrated community case management (iCCM)
By 2004, Rwanda had learned that Kenya and Uganda were using trained volunteers to screen children with fevers as a way to identify and treat cases of malaria.,, Rwanda soon followed their lead and expanded its CHW program to include management of childhood illnesses, starting with the adoption of home-based management of fever (HBMF).
Over a two-year pilot program, the health ministry tested CHW delivery of HBMF in six districts. In most locations, the proportion of children receiving treatment within 24 hours of the onset of fever had increased to more than 80 percent, and health centers were reporting fewer cases of malaria., Such early treatment is critical to the reduction of malaria fatality rates.
After the nationwide rollout of HBMF, rates of fever treatment for children under five within 24 hours of onset increased to 63 percent in 2008, 84 percent in 2009, and 89 percent in 2010.
However, the systematic association of fever with malaria led to overdiagnosis, and in 2008, the ministry piloted the training of CHWs to use rapid diagnostic tests (RDTs) for malaria in order to treat only confirmed cases of the disease. In addition, CHWs began distributing bednets and training village residents on their proper use.
At the same time that it was ramping up the nationwide HBMF campaign, the MOH looked into expanding the CHWs’ responsibilities to include integrated community case management (iCCM) of diarrhea and pneumonia in children under five. This approach aims to support the integration of delivery of treatments to children for diarrhea, pneumonia and malaria by community health workers.
Following a pilot program and a close review of Senegal’s successful iCCM implementation, the MOH approved a nationwide inclusion of iCCM into the CHW portfolio.
Just one year after the 2008 implementation of iCCM, districts saw increases in the number of children receiving treatment for diarrhea and pneumonia. These early interventions eventually translated into lower rates of severe illness and mortality; overall U5M declined by 38 percent and health facility use went down by 15 percent.
Insecticide-treated bednets
In 2000, malaria was among the leading causes of death for children under five in Rwanda, killing just over 380 per 100,000. By 2015 the malaria U5M rate was 47 per 100,000. A nationwide campaign to distribute insecticide-treated bednets was the centerpiece strategy of this achievement.
During the late 1990s and early 2000s, Rwanda distributed non-treated bednets to pregnant women; children under five; and people with HIV. In 2006, the MOH began a mass distribution campaign that issued more than three million ITNs within a year.
As a result of this campaign, the percentage of the population sleeping under ITNs increased from 13 percent in 2005 to 28 percent in 2006, and then to 59 percent in 2007. Rwanda embarked on another country-wide campaign in 2008 and 2009, distributing over six million additional ITNs and banning the importation of untreated nets.
The decline in malaria cases changed unexpectedly in 2009, when 28 of 30 districts reported sudden upticks in malaria incidence and mortality. In an important example of how Rwanda’s rigorous ongoing monitoring-and-evaluation processes could head off problems and improve long-term outcomes, the problem was swiftly identified - and traced to the bednets themselves.
While the WHO had certified the nets to be effective for three to five years, independent tests showed that the efficacy of the ITNs had actually begun to decline at only 18 months. By the end of 2009, effective ITN coverage had fallen to 24 percent.
The MOH responded by analyzing health data to identify the places where coverage rates had declined most sharply. It then strategically distributed its remaining ITNs to those areas. Using the same data, the ministry applied successfully for a Global Fund grant for another mass-distribution campaign targeting the districts at highest risk and replacing ITNs every two years. By 2010, 82 percent of homes possessed at least one mosquito net, and 70 percent of all children under five slept beneath one.
Indoor residual spraying
Indoor residual spraying (IRS) has advanced more slowly than ITNs, due to its relatively high costs. Three districts in Kigali underwent a US President’s Malaria Initiative (PMI)-supported pilot spraying program in 2007, with five additional high-transmission districts included over the following two years.
Altogether, the seven districts in the spraying program represented approximately 70 percent of Rwanda’s malaria caseload, and IRS coverage in the targeted areas ranged from 87 percent to 99 percent.
A five-district PMI-sponsored IRS campaign took place in 2011, with three-district campaigns occurring over the 2012-2014 period, and a fourth district added in 2015. All districts targeted for IRS are selected based on their relatively high rates of malaria.
A statistical analysis from the Institute for Health Metrics and Evaluation (IHME) found that 2.0 percent of the reduction in under-five deaths was attributable to insecticide-treated nets and indoor residual spraying.
Malaria diagnosis and treatment protocol updates
In 2001, Rwanda changed its first-line antimalarial treatment from chloroquine to amodiaquine and sulfadoxine-pyrimethamine (AQ+SP) based on resistance monitoring within the country.
Five years later, the MOH introduced artemisinin-based combination therapy (ACT) as the nation’s new first-line treatment for facility-based care. After health centers showed sharp drops in malaria mortality in children with this change in treatment, the MOH adopted ACT in 2007 as the first-line treatment in community settings as well.
As a result of these and other interventions, malaria’s death rate was reduced by 88 percent among children under five during the period from 2000 to 2015.

Measles vaccine
Rwanda’s campaign against measles represented one of the nation’s earliest U5M interventions and became a model for later achievements - especially in vaccine delivery.
Measles vaccine was introduced in Rwanda in 1980, and national coverage of the vaccine ranged between 74 and 89 percent prior to the 1994 genocide. By 1995, just after the genocide and destruction of the health sector, measles became one of the leading causes of death for children under five.
By 2002, measles vaccination rates had recovered to just below pre-genocide levels - 69 to 74 percent - with thousands of cases still occurring each year. In 2003, the vaccination rate increased to 90 percent and the vaccination rate has remained in the nineties since 2006.
With the rapid expansion of the vaccination program, cases of measles decreased from over 3,000 deaths in 2000 to hundreds annually by 2004, and to only six nationwide by 2008. Rwanda has set a goal to eliminate measles by 2020 - an objective no other African nation has yet achieved.
The national government has established a technical committee to consider the introduction of new vaccines, and this body supported the rollout of a measles-rubella (MR) combined vaccine in 2012. In 2013, the MOH began a program to administer the MR vaccine to all children from nine months to 15 years old. The campaign targeted sites including schools, community centers, and border stations, as well as hospitals and clinics.
The MOH used a household survey to identify and target regions with low coverage. By the time the campaign ended, 93 percent of Rwandan children had undergone a full vaccination round for measles and rubella. As a result of the MR campaign, measles deaths have plummeted to nearly zero (from a rate of 155 per 100,000 population under 5 in 2000 to 4 per 100,000 in 2015).
The success of the measles vaccination campaigns has given Rwanda an EBI template for maintaining high vaccine coverage for other diseases. Thanks to a comprehensive diphtheria-pertussis-tetanus (DPT) vaccination program from 2000 to 2015, Rwanda has seen low incidence of that disease since 2005.
Nor have there been any documented cases of polio since 2000 - again, thanks to a thoroughgoing vaccination program that reached virtually the entire population.
However, challenges remain. With the flow of refugees into Rwanda, primarily from Burundi and the Democratic Republic of the Congo, there is still a significant risk of measles outbreaks among the unvaccinated.
For more information on this regional challenge, click here.
PCV vaccination campaign
Along with other lower-respiratory infections (LRIs), pneumonia has been the leading cause of death for children under five throughout the period covered by this report. To address this, Rwanda has introduced two pneumonia vaccines - the pneumococcal conjugate vaccine (PCV) and Haemophilus influenzae type B vaccine (HiB).
The success of the vaccination introductions is a major factor in the sharp decline in LRI deaths from 2000 (720 deaths per 100,000) to 2015 (170 per 100,000 children).
In 2007, the WHO added PCV to its list of recommended routine childhood immunizations. Two years later, Rwanda became the first country in sub-Saharan Africa to deploy PCV.
Rwanda completed the rollout within five months, and vaccination coverage levels have remained at around 97 percent since 2010. In 2011, Rwanda switched from PCV-7 to the newly available PCV-13, which protected against six more strains of the bacteria, and required less cold storage space and lower incineration temperatures for waste management.
PCV is now included in the standard pediatric vaccine schedule, and CHWs gather monthly data to identify any gaps in coverage.
Rotavirus vaccination campaign
The PCV drive gave Rwanda the cold chain capacity, the monitoring capacity, and the institutional expertise to carry out similar large-scale vaccine introductions - including a new one targeting diarrhea.
A year after the PCV campaign, the laboratory at the University Teaching Hospital of Rwanda (CHUK) found that 30 percent of hospitalized pediatric patients’ stool samples tested positive for rotavirus.
In 2010, Rwanda initiated implementation strategies for the rotavirus vaccine, including further upgrades to the cold chain. In May 2012, Rwanda became the first low-income African country to introduce routine rotavirus vaccinations - and the first nation to introduce both the pneumococcal and rotavirus vaccines.
Rotavirus vaccination rates in children under one year of age increased from 50 percent in 2012 to 98 percent in 2013.
Hospital admissions for severe diarrhea in children under five declined by 49 percent between 2011 and 2013., By 2015, U5M attributable to diarrheal diseases had fallen to 106 deaths per 100,000 children under five.
Diarrhea-related hospital admissions tumbled immediately following the introduction of the vaccine among both vaccine-eligible infants (those less than one year of age) and among older children who were not eligible for the vaccine (those between the ages of one and five). This suggested an element of collective protection through reduced transmission - an important additional benefit of Rwanda’s rotavirus-vaccine rollout.
Overall, across all vaccines, statistical analysis found that 19.4 percent of the reduction in under-five deaths was attributable to vaccine interventions, including Hib vaccine (6.6 percent), PCV (5.1 percent), DTP3 (3.6 percent), rotavirus vaccine (2.6 percent) and measles first dose vaccine (1.5 percent) .
Prevention of mother-to-child transmission (PMTCT) program
In 1989, the University Teaching Hospital of Rwanda (CHUK) began testing for HIV among pregnant women. And in 1999, the MOH began Rwanda’s first prevention of mother-to-child transmission (PMTCT) program. This program included pretest HIV counseling; routine HIV testing at antenatal care visits; and prophylactic antiretroviral treatment (ART) for HIV-positive pregnant women.
To meet the high demand for PMTCT programs, Rwanda secured over $75 million in grant funding from UNICEF, the Global Fund, the Elizabeth Glaser Pediatric AIDS Foundation, and other sources to underwrite a national HIV program, including broad-scale PMTCT and ART implementation.
In 2001, the Treatment and Research on AIDS Center (TRAC) defined national goals for PMTCT in the National Strategic Plan against HIV. Rwanda enacted the plan four years later. The MOH created a new health-information management system called TRACnet, which drew upon reports submitted by CHWs via mobile phone to provide timely data on HIV cases. In 2006, a national scale-up plan was introduced, with the goal of integrating comprehensive PMTCT into community-based maternal and children’s health services.
In 2010, Rwanda introduced the Option B treatment regimen for PMTCT, which recommended triple therapy for new mothers from birth through weaning. It was criticized for being costlier than the Option A program, which ceased triple therapy one week after childbirth and had been endorsed by the WHO. The MOH believed that Option B would be most cost-effective option in the long run because it would prevent more new cases of HIV. Later studies proved these national findings correct.
When Rwanda implemented Option B, it chose to continue triple therapy for mothers for life. It was not until two years later that the WHO officially recommended this strategy (now known as Option B+) as a superior alternative to Option A.
By 2009, all health centers in Rwanda offered antenatal care, the main entry point for PMTCT treatment. Rwanda’s MTCT rates came down from 9.7 percent in 2006 to 1.8 percent in 2015. In 2011, the First Lady of Rwanda launched a campaign to eliminate MTCT in Rwanda, further marking this issue as a national priority.

Rwanda has had greater difficulty implementing early infant diagnosis (EID) - a critical tool for reducing HIV-related mortality. Without treatment, more than half of HIV-positive infants will die by the age of two.
By 2008, half of all health facilities in Rwanda were offering EID services, and 70 percent were doing so by the following year. Yet only 28 percent of children born to HIV-positive mothers were receiving EID.
The MOH organized the first National Symposium on Early Infant Diagnosis in 2009. This conference assisted the MOH in mapping out specific problems, including poor integration with maternal and child-health programs; delays in the processing of HIV tests; and lack of follow-up with caregivers.
The MOH adopted specific changes in its EID and pediatric ART programs to address these identified gaps, including adjustments of the routine immunization schedule to include HIV testing for exposed infants at six weeks, nine months and 18 months.
Even with these improvements in testing and vastly expanded access to ART, treatment of HIV-positive children remains limited. In 2013, only 60 percent of HIV-positive children up to 14 years old were receiving ART, compared to 95 percent of HIV-positive adults.
Malnutrition interventions
In 2000, 6.8 percent of Rwandan children under five were acutely malnourished and 48 percent of children were stunted. According to the 2003 MOH annual report, severe acute malnutrition was the fourth most common cause of death for children up to one year old, and the second most common cause of death for children between the ages of one and 14.
In 2009, the President’s Initiative to Eliminate Malnutrition was approved, and 30,000 CHWs were trained in identifying malnutrition in children under five, and in appropriate interventions and referral processes.
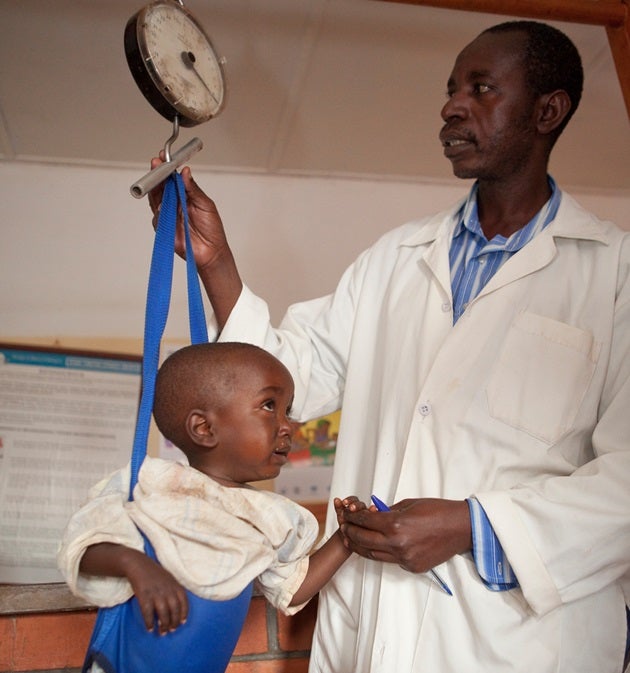
CHWs measure children at monthly community meetings. If a child misses one meeting, the CHW will follow up with a home visit. Parents of children deemed at risk based on their measurements are then invited to “kitchen demonstration activities,” where families bring in food and cook together to learn about nutrition from the CHW. Any children who are deemed malnourished are given ready-to-use therapeutic foods and referred to health facilities for treatment.
Even as Rwanda has led the region in reducing severe acute malnutrition, it has lagged in efforts to reduce chronic malnutrition and stunting. While severe acute malnutrition is the more urgent issue from an infant-mortality standpoint, Rwanda’s difficulty in addressing chronic malnutrition has U5M consequences of its own, given the impact of long-term undernourishment on children’s health, mental abilities, and future earning potential.
To better address chronic malnutrition, the MOH started a new nutrition program to provide free nutrient-rich porridge to children aged six months to two years in the nation’s poorest families.
Improvements in antenatal and neonatal care
Although Rwanda significantly outperformed its regional and global counterparts on U5M overall between 2000 and 2015, its record on neonatal mortality is considerably more modest.
The 2010 Global Burden of Disease report showed that neonatal deaths in Rwanda had increased from 22 percent of all under-five deaths in 2000 to 34 percent in 2010, even as the absolute number of neonatal deaths was decreasing. The mortality rates among infants under one month of age showed the least progress of all.
The Ministry of Health initiated weekly neonatal death audits in 40 public and faith-based health facilities in 2012. Using a standardized questionnaire, all neonatal deaths in these facilities were reported to the Ministry of Health in Kigali. Based on these findings, the MOH adopted interventions based on specific causes of death affecting newborns, the two most common being asphyxia and complications due to prematurity.
The CHW program began in 2003 to train local traditional birth attendants - who qualify to be animatrices de santé maternelle (ASMs) - to advise women to deliver in health facilities. As noted earlier, these birth attendants also serve as a third community health worker, supplementing the original male-and-female CHW pair. Over the following 15 years, the rate of facility-based delivery increased significantly. This reflects both the work of ASMs encouraging women to deliver in a health facility and the improved quality of care available at health facilities as the number of formally trained health providers grows. As the country established more nursing schools, the number of nurses and midwives nearly tripled from an estimated 3,600 in 2004 to 9,600 in 2015.
In addition, health providers now teach post-partum mothers how to breastfeed while they are still in the health facility. This practice - accompanied by the fact that over 90 percent of Rwandan women now deliver in health facilities - has resulted in a sharp rise in the proportion of children breastfed within one hour of birth, from 41 percent in 2005 to 71 percent in 2010 and 81 percent in 2015.
After finding that approximately three-quarters of newborns who died were hypothermic when admitted, the MOH began providing incubators for health facilities. In 2012, it established skin-to-skin contact, or kangaroo mother care (KMC), in its neonatal protocol for premature and low-birth-weight infants.
According to a 2012 quality of care study, 100 percent of children born in health facilities in Rwanda are immediately dried with a towel, but only about half of those infants are then placed skin-to-skin with mother or covered with a dry towel or blanket, a proportion that will need to rise if neonatal-survival outcomes are to improve.
The leading causes of neonatal mortality are now low birth weight, prematurity, and congenital heart disease - factors that will require more advanced neonatal care. An important step will be an increase in the number of women who receive the four or more antenatal-care visits recommended by the WHO.
Rates of ANC by a skilled provider have increased from 25 percent of women in 2000 to 92 percent in 2015. However, as of 2015, only 44 percent of pregnant women had four or more ANC visits.
Another area for improvement is in the use of partographs - graphical records of health indicators during the labor process, to ensure that health workers throughout a woman’s entire labor process receive an unbroken sequence of vital information. Blank partographs are available at all health facilities, and the use of partographs has been added as an indicator for performance-based financing. While partographs are used in approximately 84 percent of deliveries in health care facilities, only eight percent of them are filled in completely.,,,,
Decomposition
A decomposition analysis undertaken in collaboration with the Institute for Health Metrics and Evaluation (IHME) at the University of Washington identified the top interventions and risk factors that contributed to reductions in under-five deaths in Rwanda. These interventions and risk factors can be seen in the bottom two bars of the visualization below.
Below are some of the key takeaways from this analysis:
- Some of the largest contributors to reduction in under-five mortality were health system interventions, which were responsible for 51 percent of the reduction in under-five deaths from 2000 to 2017.
- Within this, vaccines (especially Hib vaccine, PCV, and DTP3) were a significant factor, and were attributed to 19.4 percent of the reduction.
- Beyond health system interventions, population age structure, reductions in child growth failure, and reductions in other communicable disease risk factors were also found to have significant contributions to reduction in under-five deaths.
